Abstract
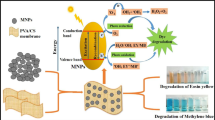
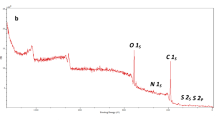
This study developed a novel nanocomposite by blending Chitosan and Polyvinyl Alcohol (Ch/PVA) with 1% Copper Oxide nanoparticles (CuO NPs), cross-linked using tetraethyl orthosilicate (TEOS). Three Ch/PVA-based nanocomposites were synthesized using CuO, Graphene Oxide (GO), and a CuO&GO combination. The research aimed to assess the nanocomposites’ efficacy in removing methyl orange (MO) dye. The X-ray diffraction analysis revealed that the fabricated CuO NPs had a monoclinic structure with an average particle size of 16.35 nm. The distinctive bands at 3222 cm−1 for the Ch/PVA composite were confirmed by Fourier-transform infrared spectroscopy (FT-IR). Scanning Electron Microscopy (SEM) results confirmed the NPs were well distributed throughout the matrix and Thermal gravimetric analysis (TGA) verified the stability of chitosan till 350 °C. The sorption experiments with methyl orange dye were carried out under different pH (2–12), dye concentration (0.025–1.0 mML−1), contact time (0–60 min), and temperature (25–55 °C) to establish the adsorbent at the lab-scale. UV–vis results showed the maximum sorption capacity (72 mM/g) achieved within 10 min with 96% dye removal. The adsorption behavior of nanocomposites was attributed to a physicochemical and monolayer adsorption process. Thermodynamic studies at 328 K confirmed that the adsorption process was spontaneous with ΔG° value − 4.2 KJmol−1. Also, ΔH° and ΔS° were found to be 11.3 and 52.2 KJmol−1 for MO adsorption, respectively. This work paves a new route for developing biodegradable, nontoxic, cost-effective, eco-friendly, and highly efficient adsorbents capable of selectively removing and recycling anionic dyes from wastewater.
Graphical Abstract







Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
T.A. Saleh, N.P. Shetti, M.M. Shanbhag, K.R. Reddy, T.M. Aminabhavi, Recent trends in functionalized nanoparticles loaded polymeric composites: an energy application. Mater. Sci. Energy Technol. 3, 515–525 (2020). https://doi.org/10.1016/j.mset.2020.05.005
I.Y. Jeon, J.B. Baek, Nanocomposites derived from polymers and inorganic nanoparticles. Materials. 3(6), 3654–3674 (2010). https://doi.org/10.3390/ma3063654
M.Z. Rong, M.Q. Zhang, Y.X. Zheng, H.M. Zeng, K. Friedrich, Improvement of tensile properties of nano-SiO2/PP composites in relation to percolation mechanism. Polymer. 42(7), 3301–3304 (2001). https://doi.org/10.1016/S0032-3861(00)00741-2
S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi, R. Kumar, Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 38(8), 1232–1261 (2013). https://doi.org/10.1016/j.progpolymsci.2013.02.003
T. Sun, J.M. Garces, High-performance polypropylene–clay nanocomposites by In‐situ polymerization with Metallocene/Clay catalysts. Adv. Mater. 14(2), 128–130 (2002). https://doi.org/10.1002/1521-4095(20020116)14. :2%3C128::AID-ADMA128%3E3.0.CO;2-7
J. Luna, A. Vílchez, (2017). Polymer nanocomposites for food packaging. In Emerging nanotechnologies in food science. Elsevier, Amsterdam, pp 119–147 https://doi.org/10.1016/B978-0-323-42980-1.00007-8
K.K. Sadasivuni, D. Ponnamma, M. Rajan, B. Ahmed, M.A.S. Al-Maadeed (eds.), Polymer nanocomposites in biomedical engineering (Springer, Heidelberg, 2019).
L. Tamayo, M. Azócar, M. Kogan, A. Riveros, M. Páez, Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Engineering: C 69, 1391–1409 (2016). https://doi.org/10.1016/j.msec.2016.08.041
L. Flandin, Y. Brechet, J.Y. Cavaillé, Electrically conductive polymer nanocomposites as deformation sensors. Compos. Sci. Technol. 61(6), 895–901 (2001). https://doi.org/10.1016/S0266-3538(00)00175-5
A. Nasir, F. Masood, T. Yasin, A. Hameed, Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 79, 29–40 (2019). https://doi.org/10.1016/j.jiec.2019.06.052
C. Tang, N. Chen, Q. Zhang, K. Wang, Q. Fu, X. Zhang, Preparation and properties of chitosan nanocomposites with nanofillers of different dimensions. Polym. Degrad. Stab. 94(1), 124–131 (2009). https://doi.org/10.1016/j.polymdegradstab.2008.09.008
A.H. Jawad, N. Shazwani, A. Mubarak, S. Sabar, Adsorption and mechanism study for reactive red 120 dye removal by cross-linked chitosan-epichlorohydrin biobeads. Compos. Part. B Eng. 75, 415–418 (2019). https://doi.org/10.5004/dwt.2019.24438
A.A. Mansur, F.P. Ramanery, L.C. Oliveira, H.S. Mansur, Carboxymethyl Chitosan functionalization of Bi2S3 quantum dots: towards eco-friendly fluorescent core-shell nanoprobes. Carbohydr. Polym. 146, 455–466 (2016). https://doi.org/10.1016/j.carbpol.2016.03.062
M.A. Nawi, A.H. Jawad, S. Sabar, W.W. Ngah, Photocatalytic-oxidation of solid state chitosan by immobilized bilayer assembly of TiO2–chitosan under a compact household fluorescent lamp irradiation. Carbohydr. Polym. 83(3), 1146–1152 (2011). https://doi.org/10.1016/j.carbpol.2010.09.044
S. Sarode, P. Upadhyay, M.A. Khosa, T. Mak, A. Shakir, S. Song, A. Ullah, Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 121, 1086–1100 (2019). https://doi.org/10.1016/j.ijbiomac.2018.10.089
Z.I. Abdeen, E. Farargy, A. F., N.A. Negm, Nanocomposite framework of chitosan/polyvinyl alcohol/ZnO: Preparation, characterization, swelling and antimicrobial evaluation. J. Mol. Liq. 250, 335–343 (2018). https://doi.org/10.1016/j.molliq.2017.12.032
A.H. Jawad, M.A. Nawi, Oxidation of cross-linked chitosan-epichlorohydrine film and its application with TiO2 for phenol removal. Carbohydr. Polym. 90(1), 87–94 (2012). https://doi.org/10.1016/j.carbpol.2012.04.066
M. Shafiq, A. Sabir, A. Islam, S.M. Khan, S.N. Hussain, M.T.Z.Z. Butt, T. Jamil, Development and performance characteristics of silane cross-linked poly (vinyl alcohol)/chitosan membranes for reverse osmosis. J. Ind. Eng. Chem. 48, 99–107 (2017). https://doi.org/10.1016/j.jiec.2016.12.025
Q. Yu, Y. Song, X. Shi, C. Xu, Y. Bin, Preparation and properties of chitosan derivative/poly (vinyl alcohol) blend film cross-linked with glutaraldehyde. Carbohydr. Polym. 84(1), 465–470 (2011). https://doi.org/10.1016/j.carbpol.2010.12.006
M. Zakeri, H. Mobedi, J. Barzin, A. Jamshidi, A. Mashak, Development of chitosan beads for controlled release of dexamethasone prepared by co-axial needle method. J. Polym. Res. 27, 1–11 (2020). https://doi.org/10.1007/s10965-020-02232-z
T. Thanyacharoen, P. Chuysinuan, S. Techasakul, P. Nooeaid, S. Ummartyotin, Development of a gallic acid-loaded chitosan and polyvinyl alcohol hydrogel composite: release characteristics and antioxidant activity. Int. J. Biol. Macromol. 107, 363–370 (2018). https://doi.org/10.1016/j.ijbiomac.2017.09.002
M.M. Hasan, M.L. Habib, M. Anwaruzzaman, M. Kamruzzaman, M.N. Khan, M.M. Rahman, (2020). Processing techniques of chitosan-based interpenetrating polymer networks, gels, blends, composites and nanocomposites. In Handbook of Chitin and Chitosan (pp. 61–93). Elsevier, Amsterdam https://doi.org/10.1016/B978-0-12-817968-0.00003-2
A.C.M. Oliveira, M.S. Santos, L.M. Brandão, N. Yerga, R.M. Fierro, J.L.G. Leite, M.S. Figueiredo, Chitosan-modified TiO2 as photocatalyst for ethanol reforming under visible light. Chem. Pap. 71, 1129–1141 (2017). https://doi.org/10.1007/s11696-016-0095-2
M. Aslam, Z.A. Raza, A. Siddique, Fabrication and chemo-physical characterization of CuO/chitosan nanocomposite-mediated tricomponent PVA films. Polym. Bull. 78, 1955–1965 (2021). https://doi.org/10.1007/s00289-020-03194-4
A. Thekkedath, S. Sugaraj, K. Sridharan, (2022). Nanomaterials in advanced oxidation processes (AOPs) in anionic dye removal. Advanced Oxidation Processes in Dye-Containing Wastewater: Volume 1 (129–165). Singapore: Springer Nature Singapore. https://doi.org/10.1007/978-981-19-0987-0_7
V. Sharma, T. Shahnaz, S. Subbiah, S. Narayanasamy, New insights into the remediation of water pollutants using nanobentonite incorporated nanocellulose chitosan based aerogel. J. Polym. Environ. 28, 2008–2019 (2020). https://doi.org/10.1007/s10924-020-01740-9
B. Tanhaei, A. Ayati, M. Sillanpää, Magnetic xanthate modified Chitosan as an emerging adsorbent for cationic azo dyes removal: kinetic, thermodynamic and isothermal studies. Int. J. Biol. Macromol. 121, 1126–1134 (2019). https://doi.org/10.1016/j.ijbiomac.2018.10.137
S. Wong, N.A.N. Yac’cob, N. Ngadi, O. Hassan, I.M. Inuwa, From pollutant to solution of wastewater pollution: synthesis of activated carbon from textile sludge for dye adsorption. Chin. J. Chem. Eng. 26(4), 870–878 (2018). https://doi.org/10.1016/j.cjche.2017.07.015
M.F. Hanafi, N. Sapawe, A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today: Proc. 31, A141–A150 (2020)
R. Raliya, C. Avery, S. Chakrabarti, P. Biswas, Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 7, 253–259 (2017). https://doi.org/10.1007/s13204-017-0565-z
C.F. Carolin, P.S. Kumar, G.J. Joshiba, Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technol. Environ. Policy. 23, 173–181 (2021). https://doi.org/10.1007/s10098-020-01934-8
U. Habiba, T.A. Siddique, J.J.L. Lee, T.C. Joo, B.C. Ang, A.M. Afifi, Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr. Polym. 191, 79–85 (2018). https://doi.org/10.1016/j.carbpol.2018.02.081
U. Habiba, T.A. Siddique, T.C. Joo, A. Salleh, B.C. Ang, A.M. Afifi, Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo Red and chromium (VI) by flocculation/adsorption. Carbohydr. Polym. 157, 1568–1576 (2017). https://doi.org/10.1016/j.carbpol.2016.11.037
J. Liu, J. Xiong, C. Tian, B. Gao, L. Wang, X. Jia, The degradation of methyl orange and membrane fouling behavior in anaerobic baffled membrane bioreactor. Chem. Eng. J. 338, 719–725 (2018). https://doi.org/10.1016/j.cej.2018.01.052
A.T. Mohammad, A.S. Abdulhameed, A.H. Jawad, Box-Behnken design to optimize the synthesis of new cross-linked chitosan-glyoxal/TiO2 nanocomposite: methyl orange adsorption and mechanism studies. Int. J. Biol. Macromol. 129, 98–109 (2019). https://doi.org/10.1016/j.ijbiomac.2019.02.025
L. Ai, J. Jiang, Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid. Chem. Eng. J. 192, 156–163 (2012). https://doi.org/10.1016/j.cej.2012.03.056
M.F. Ahmad, S. Hassan, Z. Imran, D. Mazhar, S. Afzal, S.A. Ullah, Green approach to water purification: investigating methyl orange dye adsorption using chitosan/polyethylene glycol composite membrane. J. Polym. Environ. 32, 1–19 (2023). https://doi.org/10.1007/s10924-023-02994-9
M. Patel, S. Mishra, R. Verma, D. Shikha, Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various technique. Discover Mater. 2(1), 1 (2022). https://doi.org/10.1007/s43939-022-00022-6
Z. Alhalili, Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem. 15(5), 103739 (2022). https://doi.org/10.1016/j.arabjc.2022.103739
N. Nasikhudin, I. Puspitasari, M. Diantoro, A. Kusumaatmaja, K. Triyana, Effect of blend ratio on morphology and swelling properties of PVA/chitosan nanofibers. Mater. Sci. Forum 901, 79–84 (2017)
S. Kumar, B. Krishnakumar, A.J. Sobral, J. Koh, Bio-based (chitosan/PVA/ZnO) nanocomposites film: thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 205, 559–564 (2019). https://doi.org/10.1016/j.carbpol.2018.10.108
K.S. Venkataprasanna, J. Prakash, S. Vignesh, G. Bharath, M. Venkatesan, F. Banat, G.D. Venkatasubbu, Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 143, 744–762 (2020). https://doi.org/10.1016/j.ijbiomac.2019.10.029
A. Islam, T. Yasin, I. Bano, M. Riaz, Controlled release of aspirin from pH-sensitive chitosan/poly (vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 124(5), 4184–4192 (2012). https://doi.org/10.1002/app.35392
A. Pawlak, M. Mucha, Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta. 396(1–2), 153–166 (2003). https://doi.org/10.1016/S0040-6031(02)00523-3
Y. Zhou, S. Fu, L. Zhang, H. Zhan, M.V. Levit, Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for pb (II). Carbohydr. Polym. 101, 75–82 (2014). https://doi.org/10.1016/j.carbpol.2013.08.055
C.D.T. Neto, J.A. Giacometti, A.E. Job, F.C. Ferreira, J.L.C. Fonseca, M.R. Pereira, Thermal analysis of Chitosan based networks. Carbohydr. Polym. 62(2), 97–103 (2005). https://doi.org/10.1016/j.carbpol.2005.02.022
P. Khare, A. Yadav, J. Ramkumar, N. Verma, Microchannel-embedded metal–carbon–polymer nanocomposite as a novel support for chitosan for efficient removal of hexavalent chromium from water under dynamic conditions. Chem. Eng. J. 293, 44–54 (2016). https://doi.org/10.1016/j.cej.2016.02.049
D.D. Kachhadiya, Z.V.P. Murthy, Graphene oxide modified CuBTC incorporated PVDF membranes for saltwater desalination via pervaporation. Sep. Purif. Technol. 290, 120888 (2022). https://doi.org/10.1016/j.seppur.2022.120888
S.K. Balasubramanian, L. Yang, L.Y.L. Yung, C.N. Ong, W.Y. Ong, E.Y. Liya, Characterization, purification, and stability of gold nanoparticles. Biomaterials. 31(34), 9023–9030 (2010). https://doi.org/10.1016/j.biomaterials.2010.08.012
L. Miao, C. Wang, J. Hou, P. Wang, Y. Ao, Y. Li, Y. Xu, Enhanced stability and dissolution of CuO nanoparticles by extracellular polymeric substances in aqueous environment. J. Nanopart. Res. 17, 1–12 (2015). https://doi.org/10.1007/s11051-015-3208-x
E. Dražević, K. Košutić, V. Freger, Permeability and selectivity of reverse osmosis membranes: correlation to swelling revisited. Water Res. 49, 444–452 (2014). https://doi.org/10.1016/j.watres.2013.10.029
N. Gull, S.M. Khan, O.M. Butt, A. Islam, A. Shah, S. Jabeen, M.T.Z. Butt, Inflammation targeted Chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 162, 175–187 (2020). https://doi.org/10.1016/j.ijbiomac.2020.06.133
N. Gull, S.M. Khan, M.T.Z. Butt, S. Khalid, M. Shafiq, A. Islam, R.U. Khan, In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: a preclinical study. RSC Adv. 9(53), 31078–31091 (2019). https://doi.org/10.1039/C9RA05025F
A. Rasool, S. Ata, A. Islam, Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 203, 423–429 (2019). https://doi.org/10.1016/j.carbpol.2018.09.083
E.N. Zare, A. Motahari, M. Sillanpää, Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ. Res. 162, 173–195 (2018). https://doi.org/10.1016/j.envres.2017.12.025
X. Xu, M. Tian, L. Qu, S. Zhu, Graphene Oxide/Chitosan/Polyvinyl-Alcohol composite sponge as effective adsorbent for dyes. Water Environ. Res. 89(6), 555–563 (2017). https://doi.org/10.2175/106143016X14609975746127
N. Mohammadi, H. Khani, V.K. Gupta, E. Amereh, S. Agarwal, Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 362(2), 457–462 (2011). https://doi.org/10.1016/j.jcis.2011.06.067
S. Rahim, R. Ullah, M. Tuzen, S. Ullah, A. Sarı, T.A. Saleh, Synthesis of alumina-carbon framework for efficient sorption of methyl orange from wastewater with factorial design and mechanisms. Groundw. Sustainable Dev. 22, 100950 (2023). https://doi.org/10.1016/j.gsd.2023.100950
L. Hou, X. Zhang, H. Liu, H. Zheng, B. Niu, J. Zheng, J. Fu, Rigid-flexible coupled polyphosphazene supported polyurethane foam for efficient and selective adsorption of anionic dyes from water. Colloids Surf., a 669, 131483 (2023). https://doi.org/10.1016/j.colsurfa.2023.131483
O. Moradi, A. Pudineh, S. Sedaghat, Synthesis and characterization Agar/GO/ZnO NPs nanocomposite for removal of methylene blue and methyl orange as azo dyes from food industrial effluents. Food Chem. Toxicol. 169, 113412 (2022). https://doi.org/10.1016/j.fct.2022.113412
A.H. Faris, K.J. Hamid, A.M. Naji, M.K. Mohammed, O.A. Nief, M.S. Jabir, Novel Mo-doped WO3/ZnO nanocomposites loaded with polyvinyl alcohol towards efficient visible-light-driven photodegradation of methyl orange. Mater. Lett. 334, 133746 (2023). https://doi.org/10.1016/j.matlet.2022.133746
B.S. Rathore, N.P.S. Chauhan, M.K. Rawal, S.C. Ameta, R. Ameta, Chitosan–polyaniline–copper (II) oxide hybrid composite for the removal of methyl orange dye. Polym. Bull. 77(9), 4833–4850 (2020). https://doi.org/10.1007/s00289-019-02994-7
H. Ali, T.M. Tiama, A.M. Ismail, New and efficient NiO/chitosan/polyvinyl alcohol nanocomposites as antibacterial and dye adsorptive films. Int. J. Biol. Macromol. 186, 278–288 (2021). https://doi.org/10.1016/j.ijbiomac.2021.07.055
S. Kader, M.R. Al-Mamun, M.B.K. Suhan, S.B. Shuchi, M.S. Islam, Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and ag doped TiO2 photocatalysts. Environ. Technol. Innov. 27, 102476 (2022). https://doi.org/10.1016/j.eti.2022.102476
S. Yildirim, B. Isik, V. Ugraskan, Methyl orange dye sequestration using polyaniline nanotube-filled sodium alginate bio-composite microbeads. Mater. Chem. Phys. (2023). 128083https://doi.org/10.1016/j.matchemphys.2023.128083
A.A. Hambisa, M.B. Regasa, H.G. Ejigu, C.B. Senbeto, Adsorption studies of methyl orange dye removal from aqueous solution using Anchote peel-based agricultural waste adsorbent. Appl. Water Sci. 13(1), 24 (2023). https://doi.org/10.1007/s13201-022-01832-y
L. You, C. Huang, F. Lu, A. Wang, X. Liu, Q. Zhang, Facile synthesis of high performance porous magnetic chitosan-polyethylenimine polymer composite for Congo red removal. Int. J. Biol. Macromol. 107, 1620–1628 (2018). https://doi.org/10.1016/j.ijbiomac.2017.10.025
X. Jiao, Y. Gutha, W. Zhang, Application of chitosan/poly (vinyl alcohol)/CuO (CS/PVA/CuO) beads as an adsorbent material for the removal of pb (II) from aqueous environment. Colloids Surf., B 149, 184–195 (2017). https://doi.org/10.1016/j.colsurfb.2016.10.024
E.H. Cavalcante, I.C. Candido, H.P. de Oliveira, K.B. Silveira, Víctor. de Souza, T. Álvares, E.C. Lima, G. Simões Dos Reis, 3-Aminopropyl-triethoxysilane-functionalized tannin-rich grape biomass for the adsorption of methyl orange dye: synthesis, characterization, and the adsorption mechanism. ACS omega 7(22), 18997–19009 (2022)
T.A. Saleh, Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of pb (II): from surface properties to sorption mechanism. Desalination Water Treat. 57(23), 10730–10744 (2016). https://doi.org/10.1080/19443994.2015.1036784
T.A. Saleh, (2022). Kinetic models and thermodynamics of adsorption processes: classification. In Interface Science and Technology (Vol. 34, pp. 65–97). Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-849876-7.00003-8
A. Villabona-Ortíz, C.N. Tejada-Tovar, R. Ortega-Toro, Modelling of the adsorption kinetics of chromium (VI) using waste biomaterials. Revista Mexicana De Ingeniería Química. 19(1), 401–408 (2020). https://doi.org/10.24275/rmiq/IA650
K. Kaur, R. Jindal, Self-assembled GO incorporated CMC and Chitosan-based nanocomposites in the removal of cationic dyes. Carbohydr. Polym. 225, 115245 (2019). https://doi.org/10.1016/j.carbpol.2019.115245
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918). https://doi.org/10.1021/ja02242a004
S. Asuha, X.G. Zhou, S. Zhao, Adsorption of methyl orange and cr (VI) on mesoporous TiO2 prepared by hydrothermal method. J. Hazard. Mater. 181(1–3), 204–210 (2010). https://doi.org/10.1016/j.jhazmat.2010.04.117
T. Ahamad, M. Naushad, T. Al-Shahrani, N. Al-Hokbany, S.M. Alshehri, Preparation of Chitosan based magnetic nanocomposite for tetracycline adsorption: kinetic and thermodynamic studies. Int. J. Biol. Macromol. 147, 258–267 (2020). https://doi.org/10.1016/j.ijbiomac.2020.01.025
F. Ali, N. Ali, I. Bibi, A. Said, S. Nawaz, Z. Ali, M. Bilal, Adsorption isotherm, kinetics and thermodynamic of acid blue and basic blue dyes onto activated charcoal. Case Stud. Chem. Environ. Eng. 2, 100040 (2020)
J. Wang, X. Guo, Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere. 258, 127279 (2020). https://doi.org/10.1016/j.chemosphere.2020.127279
X. Chen, M.F. Hossain, C. Duan, J. Lu, Y.F. Tsang, M.S. Islam, Y. Zhou, Isotherm models for adsorption of heavy metals from water-A review. Chemosphere 307, 135545 (2022). https://doi.org/10.1016/j.chemosphere.2022.135545
B. Tanhaei, A. Ayati, E. Iakovleva, M. Sillanpää, Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 164, 3621–3631 (2020). https://doi.org/10.1016/j.ijbiomac.2020.08.207
Acknowledgements
The authors thank the chemistry department and physics department of COMSATS University Islamabad for their invaluable support throughout our research journey. Also, we would like to extend our sincere appreciation to the Sensing and Catalysis Lab (COMSATS University Islamabad) for their invaluable support and collaboration throughout this research work. Thank you, COMSATS University Islamabad, for being such an integral part of this endeavor.
Funding
There is no funding found with authors.
Author information
Authors and Affiliations
Contributions
Sumra Afzal: Designing of experiment, synthesis of material and Characterization and analysis of the material, Syed Amin Ullah: Figures, review and formal analysis, Zahid Imran and Safia Hassan: Methodology Conceptualization, Writing editing and review, Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests that can influence the work reported in this research paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afzal, S., Hassan, S., Imran, Z. et al. Chitosan Based Polymer Membrane Modified with CuO/Graphene Oxide Nanoparticles: Novel Synthesis, Characterization and Enhanced Methyl Orange Removal. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03008-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03008-4