Abstract
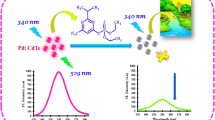
Polyindole is considered an excellent conducting polymer with interested properties for different applications. A novel polyindole (PIn)/CuInS (CIS)/ZnS quantum dots (QDs) nanocomposite was synthesized via in situ polymerization of PIn in presence of CIS/ZnS QDs. By investigating the effect of CIS/ZnS QDs on optical properties of PIn, it was found that the optical band gaps of PIn, CIS/ZnS QDs, and PIn/CIS/ZnS QDs nanocomposite were 3.24 eV, 4.68 eV and 3.44 eV, respectively. From the luminance spectra, it was observed that emission peaks of PIn at 442 and 468 nm are independent of the excitation wavelength with the highest intensity at excitation wavelength of 380 nm. However, the luminance spectrum of PIn/CIS/ZnS QDs nanocomposite exhibited a quenching peak for CIS/ZnS QDs while the intensity of PIn peak was enhanced. High resolution of transmission electron microscope image of CIS/ZnS QDs revealed nanocrystals with a size of 3–4.5 nm and lattice space of 0.2 nm. PIn/CIS/ZnS QDs nanocomposite as the fluorescent probe was employed for sensing different concentrations of Pb2+ from 5 to 50 ppb. The reaction between PIn/CIS/ZnS QDs and Pb2+ was slightly quenched and fixed after 90 min. The emission peak was reduced gradually with increasing concentration of lead via photo-induced electron transfer or ion exchange mechanism. The value of correlation coefficient (R2) was 0.99, the sensitivity was 0.0041 ppb−1 and limit of detection value was 4.48 ppb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The nanotechnology has opened an intensive advanced approach to prepare novel nanostructures based on their properties including the small size with high surface area and their characteristic physical and chemical properties [1, 2]. Nanostructures involved nanowires, nanoparticles and nanotubes can be classified to organic and inorganic nanocomposites. Physicochemical advantages of these nanostructures like their thermal and electrical conductivity, optical sensitivity and also high surface area provide them to be applied in sensing, storage and other optoelectronic devices. Additionally, for the advanced applications in nanoelectronics, energy storage, water treatment and nanomedicine there are extensive works turned to formation of new nanocomposite materials [3,4,5,6,7,8,9,10,11,12,13]. Novel nanocomposites formed between conjugated conducting organic polymers and inorganic nanoparticles with unique chemical and physical advantages is the main object of recent advanced technologies [14,15,16].
Nowadays, conducting polymers and quantum dots (QDs) based nanocomposites have drawn a great attention toward electronic devices such as biosensors, sensors, and energy storage. Conducting polymers can be applied for the fabrication of optoelectronic applications due to their unique electrical and optical properties [17,18,19,20,21]. There are different conjugated polymers with π-electrons such as polyaniline, polypyrrole, polythiophenes and polyindole (PIn) [22,23,24]. Among the conducting polymers, PIn has high redox activity, good thermal stability, low toxicity, high storage ability, high conductivity and low rate of degradation in comparison with those of polyaniline, polypyrrole, due to the presence of pyrrole and benzene rings in its structure [25]. Nanocomposites of PIn have been reported with metal oxides, metal nanoparticles, graphene and carbon nanotubes [26,27,28,29].
QDs are zero dimensional nanocrystals that differ from the bulk materials and have certain number of electrons in discrete quantized energy levels [30]. They have unique optical properties like high photostability, a wide range of excitation wavelength and high tunable emission [31]. Consequently, they can be applied in light emitting diode, bioimaging, sensors, supercapacitors and solar cells [32,33,34,35,36,37]. Copper indium sulfide (CIS) QDs are ternary I–III–VI nanocrystals having tunable fluorescence ranged from the visible to near-infrared spectral region based on their sizes and Cu/In ratios [38,39,40].
Ramesan et al. synthesized nanocomposite of PIn with different concentrations of CuS via in situ polymerization. They found that an increase in ac electrical conductivity and dielectric properties of the composite accompanied the increase of molar concentration of CuS nanoparticles that induced the polarization of PIn [19]. Ajeet Verma et al. prepared nanocomposite of PIn/CdS/rGO by in situ chemical oxidation, and found that the absorbance of nanocomposite is higher than that of pure PIn due to quantum confinement effect that caused by size reduction of CdS nanoparticles [18].
Pb, Cd, Hg and other heavy metals have high toxicity, bioaccumulation in human body and other living organisms and carcinogenicity. Pb pollution can be found in water, food and soil, also exists in electronic applications and batteries. Pb2+ and other heavy metals can be detected by electrochemical and fluorescent sensors for environmental monitoring in ppb level [41,42,43]. There are two motivations for this work. The first one is to synthesize of a new nanocomposite from PIn as a conducting polymer and CIS QDs. The second one is to apply this nanocomposite as a probe for Pb ions. To the best of our knowledge, there are no reports on the synthesis and characterization of PIn/CIS/ZnS QDs nanocomposite. This work aims to develop PIn/CIS/ZnS QDs nanocomposite for Pb ions detection through an in situ polymerization of PIn with CIS/ZnS QDs besides investigating the effect of QDs on structural, optical, morphological and luminance properties of PIn with different two ratios of QDs. Moreover, the interaction mechanism between PIn and CIS/ZnS QDs is proposed. This highly luminance PIn/CIS/ZnS QDs nanocomposite is applied as a fluorescent sensor for determination of Pb2+ ion concentrations in ppb level and the sensitivity, correlation coefficient and detection of limit are measured and calculated.
2 Materials and Methods
2.1 Materials
Copper chloride (99%), indium (III) chloride tetrahydrate (97%) and 3-mercaptopropionic acid (MPA) (99%) were purchased from Across Organic Company. Sodium sulfide and ammonium persulphate (APS) were received from Chem-lab. Zinc acetate dihydrate (98.5%) was supplied from Oxford Company. Ethanol (99.9%) was obtained from International Company for Sup. & Med. Industries. Isopropanol (99.5%) was bought from Alalamia Company. Hydrochloric acid was purchased from Fischer Company. Sodium hydroxide was brought from Carlo Erba. Indole (99%) was received from Cambrian chemicals. Sodium dodecyl sulphate (SDS) and chloroform (99.4%) were supplied from Fischer and Sigma Aldrich, respectively. Lead standard (1000 ppm) was purchased from Carlo Erba, Milli Q Milli pore ultrapure water.
2.2 Synthesis of CIS/ZnS QDs
Simply, 0.06 mM of InCl3·4H2O was dissolved in 10 mL deionized water with 0.045 mM of MPA. In a separate flask, 0.01 mM of CuCl2 and 0.0225 mM of MPA were mixed and the pH was adjusted to 9.0 by 1 M NaOH to obtain a clear solution. The above two mixtures were mixed with a vigorous stirring (800 rpm) for 2 min to produce molar ratio of Cu to In of 1:6. After that, 0.25 mmol of Na2S was injected into the above mixture with a vigorous stirring for 5 min. The final mixture was heated to 90 °C under reflux for 30 min and 1 mL of 0.09 M zinc acetate dihydrate was injected drop wisely for 5 min to produce core shell of CIS/ZnS QDs.
2.3 Synthesis of PIn
Two PIn samples in the powder form were polymerized with two concentrations of indole. The first PIn1 sample was prepared by dissolving 0.085 M indole in 10 mL chloroform. In another flask, SDS as a surfactant with 0.15 M was dissolved in 20 mL of 4:1 (chloroform to water) and stirred for 20 min. The SDS solution was added to the indole flask with a continuous stirring and finally the oxidizing agent of 0.098 M APS dissolved in 20 mL water and then added dropwise during 30 min to the above mixture and stirred with 800 rpm at room temperature for 24 h. The second PIn2 sample was prepared by dissolving 0.425 M indole in 10 mL chloroform. In addition, 0.19 M SDS was dissolved in 40 mL of 4:1 (chloroform to water) and also stirred for 20 min. The SDS solution was added to the indole solution with a continuous stirring and then 0.098 M APS dissolved in 20 mL water was added dropwise during 30 min to the above mixture and stirred at 800 rpm at room temperature for 24 h. The PIn powders were precipitated and separated and washed several time by deionized water and then dried at 70 °C in an oven. PIn1 and PIn2 samples were obtained with ratios of 1/1.74/1.1 and 1/0.44/1.03 from indole/SDS/APS, respectively.
2.4 Synthesis of PIn/CIS/ZnS QDs
Two nanocomposites of PIn and CIS/ZnS QDs were synthesized by in situ polymerization method using two concentrations of indole with two volumes of CIS/ZnS QDs. The first PIn/CIS/ZnS QDs1 nanocomposite solution was prepared by mixing the indole and CIS/ZnS QDs solutions. CIS/ZnS QDs (6 mL) solution was dispersed with 0.15 M SDS solution and ultrasonicated for 15 min. This solution was added to 0.085 M indole and finally the reaction was initiated by adding 0.098 M APS through 30 min. The second PIn/CIS/ZnS QDs2 solution was dispersed of 30 mL of CIS/ZnS QDs in 0.19 M SDS solution and ultrasonicated at 40 kHz for 15 min then was added to 0.425 M and finally the reaction was initiated by adding 0.438 M APS through 30 min. The reactions were left under stirring at 600 rpm speed for 24 h and then the PIn/CIS/ZnS nanocomposites were separated, washed several times with deionized water and left for dryness in oven at 70 °C to obtain the two nanocomposites PIn CIS/ZnS QDs1 and PIn CIS/ZnS QDs2.
2.5 Characterization Techniques
To study the structural, optical, crystalline and morphological properties of Pin, CIS/ZnS QDs, and their nanocomposites, UV–Vis, fluorescence, FTIR spectra and XRD and HRTEM, respectively were investigated. The structural identifications of PIn, CIS/ZnS QDs and their nanocomposites were confirmed by Fourier Transforms Infrared spectroscopic (Spectrum BX 11 Infrared spectrometer FTIR LX 18–5255 Perkin Elmer). To obtain the FTIR spectra of samples of the QDs and the nanocomposite, the powder forms were grinded with KBr powder.
UV–Vis spectra in the range from 200 to 700 nm for 200 µL for CIS/ZnS QDs diluted with 3 mL deionized water and 200 µL of PIn and nanocomposite in 2 mL of DMF to measure absorbance were measured by UV–visible spectrometer (Thermo scientific evolution 300-USA). Perkin Elmer LS 55 fluorescence spectrophotometer was used to study the emission spectra of the prepared PIn, nanocomposites and QDs, where the excitation and emission slits were 10.0 nm.
The crystallography of the prepared CIS/ZnS QDs and PIn and their nanocomposites were investigated using XRD technique (X-ray 6100 Shimadzu-Japan using copper characteristic wavelength = 1.54 A°). The 2θ angles of the diffractometer were scanned from 10° to 60° with a scan rate of 12°/min. The morphological property was studied using HRTEM images and collected by using JEOL JEM 2100F microscope at an accelerating voltage of 200 kV. Samples for HRTEM were prepared by dispersing the dried powder of the QDs, PIn and a certain composite in ethanol and allowing a drop to dry onto a 3 mm diameter of carbon coated fine copper grid and this grid was dried in air before imaging.
2.6 PIn/CIS/ZnS QDs Nanocomposite as Pb+2 Ions Sensor
A stock solution of 5 ppm of Pb2+ was prepared by dissolving 50 µL of 1000 ppm of Pb2+ in 10 mL of ultrapure water. The different concentrations of Pb2+ (5 ppb to 50 ppb) were prepared by proper dilution of the stock. PIn/CIS/ZnS QDs (45 µL) were added to 50 ppb of Pb2+ and diluted to 10 mL ultrapure water and detected at different times (0–120 min) using photoluminescence at excitation wavelength of 380 nm. Therefore, the calibration curve was carried out by measuring different concentrations of Pb2+ which added to PIn/CIS/ZnS QDs at the optimum time of 90 min.
The slope of calibration curve was calculated based on the following equation [44]:
where F° and F represent the PL intensity of PIn/CIS/ZnS QDs in the absence and presence of Pb2+, S and b refer to slope and the intercept of the calibration curve. CPb2+ is the concentration of lead. The quenching efficiency (QE) was determined using Eq. (2) [34].
The limit of detection of Pb2+ (LOD) was estimated from the following equation [44]:
where SD and S are standard deviation and slope, respectively.
3 Results and discussion
3.1 Structural properties of PIn and PIn/ CIS/ZnS QDs nanocomposite
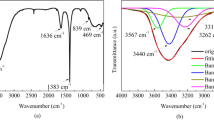
The FTIR spectra of the pristine PIn, CIS/ZnS QDs, and the nanocomposite are investigated as shown in Fig. 1. The absorption bands at ϑ 3405 cm−1 and 2934 cm−1 are corresponded to N–H stretching and CH bond of indole, respectively [45]. The characteristic bands at ϑ1629 and 1472 cm−1 belong to C–C stretching of benzenoid ring and C–N stretching, respectively. The appearance of the absorption peak at ϑ 735 cm−1 indicates out of plane benzene ring deformation [46, 47]. The presence of N–H stretching peak at ϑ 3405 cm−1 proves that the polymerization does not occur at N–H.
According to CIS/ZnS QDs, the FTIR spectrum presents the intense band at ϑ 3400 cm−1 assigned to the stretching vibration peak of O–H of MPA. Moreover, the absorption peak at ϑ 1586 cm−1 is referred to the OH groups in plane. The absorption band at ϑ 1701 cm−1 is due to the C=O stretch of carboxyl groups in CIS/ZnS QDs. The characteristic peaks at ϑ 1271 cm−1 and 2962 cm−1 are assigned to C–O–H stretching and –CH groups of capped QDs, respectively. The absorption band at ϑ 680 cm−1 is related to C–S groups of MPA attached and covered CIS/ZnS QDs [48,49,50]. To confirm the formation of nanocomposite, the broader absorbance bands at ϑ 3450 and 3431 cm−1 are appeared due to the overlapping between N–H stretching of indole moieties and OH groups of MPA. The higher intensity and sharper peak that appear at ϑ 1705 cm−1 is concerned to carbonyl groups of MPA as a capping agent. The peaks at ϑ 816 and 644 cm−1 are corresponded to CH bending and CuS stretching bond, respectively [19]. Furthermore, N–H band of composite is found to be small and broaden with a higher wavenumber from 3405 to 3450 cm−1 indicating the interaction of QDs with the indole segments. From the IR spectrum of nanocomposite, it is observed that the small peak of the QDs is found to be located at ϑ 525 cm−1 which confirmed the presence of metal–oxygen bond in the PIn.
3.2 Absorption Properties of Polyindole, CIS/ZnS, Polyindole/CIS Nanocomposite
The absorption spectra of PIn, CIS/ZnS QDs and PIn/CIS/ZnS QDs nanocomposites are presented in Fig. 2. Pure PIn shows an absorption band in the range from ~ 260 to 400 nm and having λmax at ~ 280 nm, attributed to the conjugation of the benzene ring in the indole unit [17, 51]. It is observed that major peak of absorption appears at 280 nm is ascribed to π–π* transition in benzenoid rings and at 400 nm for n–π* excitations where the incoming photons possess enough energy to excite electrons from valance band resulting into optical transition from valance band to conduction band. According to PIn 2, the absorption peaks are observed at 270, 306, 336, and 400 nm attributed to π–π* transition of the indole ring and n–π* transition. A small peak appears at 420 nm is attributed to polaronic transition as depicted in Fig. 2 [28, 52]. The main excitonic absorption peak in the CIS/ZnS QDs is not as clear as displayed in the inset of Fig. 2. The lack of excitonic peak in the absorption spectrum is due to long tail on the low-energy side of CIS and the wide size distribution of the CIS/ZnS QDs [53]. According to PIn/CIS/ZnS QDs, there are blue shifts in the peaks due to interaction between PIn and CIS/ZnS QDs with an enhancement in the polaronic transition and n–π* transition.
In order to describe the band gap energy of conducting amorphous PIn and PIn/CIS/ZnS QDs nanocomposite to estimate the photophysical properties, Tauc’s plot was used to calculate the optical band gap energy by the following equation [54, 55]:
where \(\alpha \) is the energy-dependent absorption coefficient, A is the absorbance, and d is the thickness of specimen. We apply the Tauc’s relation by creating curves between \({(\alpha h\nu )}^{2}\) and photon’s energy by following the equation [54, 55]:
where h is the Planck’s constant, B is a constant, \({E}_{g}\) is the optical band gap energy, n factor based on the electron transition nature and equal to ½ for direct transition band gaps, 2 for indirect transition, 3/2 for direct allowed and 3 for indirect forbidden band gap. It is found that the result of optical band gap of PIn is 3.24 eV, while the optical band gap value of CIS/ZnS QDs is 4.68 eV which is larger than PIn as illustrated in Fig. 3a and b. Furthermore, the values of the band gaps of the nanocomposite PIn/CIS/ZnS QDs 1 and PIn CIS/ZnS QDs 2 are 3.47 and 3.44 eV, respectively (Fig. 3c, d). The reduction of optical energy band gap of CIS/ZnS QDs after the incorporation into PIn is due to the intermediate transition of electrons from CIS/ZnS QDs to PIn.
3.3 Crystallinity Studies of PIn, CIS/ZnS QDs and Their Nanocomposite
PIn shows two reflection peaks in the XRD pattern as presented in Fig. 4. The first one is a broad peak at 21° and another one at 26.2° is owed to the amorphous nature [56]. It is noted that the polymeric materials usually present broad diffraction peak and this broad peak confirms the polymerization of indole. The second peak at 26.2° validates the partial crystalline nature of PIn. Due to Debye–Scherrer theory, the broadening of XRD patterns of the nanocrystals is resulting from the reduction of size of these crystals. On the other hand, the XRD pattern of CIS/ZnS QDs confirms the tetragonal chalcopyrite structure and likes CIS bulk structure. The reflection peaks positioned at 27.5°, 45.5°, and 53.4° are assigned to the (111), (220), and (311) lattice planes, respectively, and this is matched well with the XRD cards (JCPDS 32-0339, CuInS2) [57,58,59,60]. According to the nanocomposite, the additional peaks appear at 19°, 20°, 25°, 28°, 29°, 31°, 35°, 38° and 43.9° are attributed to the peak of the polymer and the characteristic peak of CIS/ZnS QDs [19]. The crystalline nature of composite is due to the coordination interaction between vacant orbitals of Cu in CIS and the lone-pair electrons of nitrogen of PIn, leading to a closely packed chain. By applying Scherrer’s equation; it is found that the crystallite size of PIn is 5.06 nm while the size of nanocomposite is increased to 8.4 nm, which proved that the CIS/ZnS QDs embedded with PIn matrix [18].
3.4 Morphological Properties
HRTEM images illustrated in Fig. 5 display the photographs of the synthesized PIn, CIS/ZnS QDs and PIn/CIS QDs. PIn images illustrate multilayers transparent sheets. TEM images reveal that the as-prepared CIS/ZnS QDs are highly crystalline. The size of the nanocrystals is estimated in a range of 3–4.5 nm. The values of the inter-planar spacing is 0.2 nm which correspond to (220) planes of CIS/ZnS QDs [39, 57,58,59,60,61]. From the HRTEM image, the agglomerated spherical particles of the prepared CIS/ZnS QDs are shown in Fig. 5. However, the CIS/ZnS QDs embedded in irregular and nonuniform with the polyindole matrix are visible from TEM image of polyindole/CIS/ZnS QDs nanocomposite [62, 63].
3.5 Luminance Property of PIn, CIS/ZnS QDs and PIn/CIS/ZnS QDs
PL spectra recorded for dilute solutions of PIn, CIS/ZnS QDs and PIn/CIS/ZnS QDs in DMF at different excitation wavelengths viz. 250, 300, 320, 350, 380, 400, and 420 nm are depicted in Fig. 6. The full width at half-maximums (FWHM) of PL emissions are broad with Stokes shift, suggesting that the recombination of excited electron–hole pair is occurred within the intraband. From these spectra, it is observed that emission peaks of PIn at 442 and 468 nm are independent of the excitation wavelength with the highest intensity at excitation wavelength 380 nm owing to transition of benzenoid groups that responsible for PL emission as shown in Fig. 6A. The emission spectra of PIn refer to π–π*, n–π* and polyronic transition. Moreover, this excitation-independent emission indicates a uniformity of both the quantum size and the surface states.
Figure 6B shows independent emission peaks at 640 nm PL spectra for CIS/ZnS QDs with different excitation wavelengths from 350 to 550 nm. The maximum excitation at 350 nm is declined by increasing the excitation wavelength. This is due to the high bandgap of the CIS/ZnS QDs which can be excited by higher energy photons [53]. At the excitation wavelength of 350 nm, the intensity of PL attains the maximum while for the excitation wavelengths from 450 to 550 nm, the emission peaks is reduced and have an obvious red shift. This can be interpreted based on the excitation of CIS core only not the ZnS shell [64, 65].
On the other hand, it is noted that the PL of nanocomposite PIn/CIS/ZnS QDs shows a completely quenching of PL spectrum of CIS/ZnS QDs and appeared peaks of PIn with higher excitation of 380 nm. In the PIn/CIS/ZnS QDs, the PL intensity is enhanced compared to PIn pure. This could be attributed to the in situ capping process during polymerization approach or it may be the result of electronic transition from CIS/ZnS QDs to PIn as expected from the Tauc's plot relation and increase the delocalization length of singlet exciton and polyronic transitions.
Because the PL intensity depends on the number of absorbed photons, the PL intensity enhances with increasing excitation wavelengths from 250 to 380 nm, and then decreased with increasing excitation wavelengths from 400 to 420 nm. It is recognized that shorter wavelengths with higher photon energies have high absorption coefficients and lead to increase thermalization losses and Auger assisted recombination on the surface of the QDs. Consequently, the emission intensity is reduced in the range from 400 to 420 nm. On the other hand, long-wavelength photons have longer penetration depths. Therefore, a higher fraction of long-wavelength photons is absorbed deeper in the QDs domain relative to the short-wavelength photons [66,67,68,69,70,71].
The schematic representation and energy levels diagram of the formation of PIn/CIS/ZnS QDs nanocomposite is given in Scheme 1. It is suggesting that the QDs confine inside the PIn chains, which explains the disappearance of the emission spectrum of the QDs. From the IR spectrum the formation bond of metal–oxygen bond between the QDs and PIn was suggested. Then emission spectrum of PIn/CIS/ZnS QDs proposed by the absorption of 380 nm to excite the electron from VB to CB and a recombination process occurred and the emission peak at 442 nm is illustrated in the energy band diagram of Scheme 1b [66,67,68,69,70,71].
3.6 Lead Ion Detection by PIn/CIS/ZnS QDs Nanocomposite
Figure 7 illustrates the incubation times of lead ions with PIn/CIS/ZnS QDs. The reaction between the two PIn/CIS/ZnS QDs and Pb2+ is occurred slowly and the PL intensity is quenched gradually up to 30 min and then slightly quenched till 90 min. The PL is fixed until 120 min and consequently, the optimal incubation time of 90 min is employed in further experiments. The linearity, sensitivity, dynamic range and the detection limit of PIn/CIS/ZnS QDs sensor in the presence of series of concentrations of Pb2+ (5–50 ppb) are determined using PL property. It is observed that by increasing concentration of lead ions, the PL intensity is gradually decreased as shown in Fig. 8. The PL quenching efficiency of PIn/CIS/ZnS QDs is related to lead ion concentration as presented in inset of Fig. 8.
It is noticed that the lead ions have a good affinity towards PIn/CIS/ZnS QDs with a linear relationship between the change of PL intensity (QE) and lead ions concentration ranging from 5 to 50 ppb. As known the ligands are ions or molecules that donate a pair of electrons to create a covalent bond with metal ions. Moreover, nitrogen and sulfur ligands have stronger affinity for lead metal ions than oxygen ligands. So the proposed PL quenching (turn-off) mechanism of chelated PIn/CIS/ZnS QDs and lead ions may be due to photo-induced electron transfer or ion exchange between lead and copper ion [72,73,74,75,76]. As a result of calculations from the calibration curve, it is found that the value of correlation coefficient (R2) is 0.99, the sensitivity result is 0.0041 ppb−1 and limit of detection value is 4.48 ppb. Compared to the previous works CIZS QDs was detected pb+2 at range of 20 to 80 µM and the limit of detection was 518 nM for pb+2 and the sensitivity and correlation coefficient was 0.05 µM−1and 0.98, respectively [76].
4 Conclusion
PIn/CuInS (CIS)/ZnS QDs were prepared by an in situ polymerization of PIn in presence of CIS/ZnS QDs. The Eg of PIn was 3.24 eV, while the Eg of CIS/ZnS QDs was 4.68 eV. On the other hand, the value of the band gap of the PIn/CIS/ZnS QDs was 3.44 eV. The emission peaks of PIn at 442 and 468 nm were independent of the excitation wavelength with the highest intensity at excitation wavelength of 380 nm. It is interested that the luminance spectrum for PIn/CIS/ZnS QDs quenched the peak of CIS/ZnS QDs, while the peak of PIn became high intense. The interaction between PIn and CIS/ZnS QDs anticipated that CIS/ZnS QDs were confined in the PIn matrix, which clarified the disappearance of the emission spectrum of the QDs. The lead sensing had good sensitivity of 0.0041 ppb−1, detection limits of 4.48 ppb and the dynamic linearity of 5 to 50 ppb for lead. Thus, the other sorts of sensors could benefit from the use of these unique polymer-quantum dots. Further studies in the future include the structure and interference of this nanocomposite will be investigated.
References
Y. Xiao, C.M. Li, Nanocomposites: from fabrications to electrochemical bioapplications. Electroanalysis 20(6), 648–662 (2008)
M. Nasrollahzadeh, Z. Issaabadi, M. Sajjadi, S.M. Sajadi, M. Atarod, Types of nanostructures. Interface Sci. Technol. 28, 29–80 (2019)
S. Zinatloo-Ajabshir, S.A. Heidari-Asil, M. Salavati-Niasari, Rapid and green combustion synthesis of nanocomposites based on Zn–Co–O nanostructures as photocatalysts for enhanced degradation of acid brown 14 contaminant under sunlight. Sep. Purif. Technol. 280, 119841–119852 (2022)
H. Etemadi, S. Afsharkia, S. Zinatloo-Ajabshir, E. Shokri, Effect of alumina nanoparticles on the antifouling properties of polycarbonate-polyurethane blend ultrafiltration membrane for water treatment. Polym. Eng. Sci. 61, 2364–2375 (2021)
S. Zinatloo-Ajabshir, Z. Salehi, M. Salavati-Niasari, Preparation, characterization and photocatalytic properties of Pr 2 Ce 2 O 7 nanostructures via a facile procedure. RSC Adv. 6, 107785–107792 (2016)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Preparation of nanocrystalline cubic ZrO2 with different shapes via a simple precipitation approach. J. Mater. Sci. 27, 3918–3928 (2016)
K. Mahdavi, S. Zinatloo-Ajabshir, Q.A. Yousif, M. Salavati-Niasari, Enhanced photocatalytic degradation of toxic contaminants using Dy2O3–SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach. Ultrason Sonochem. 82, 105892–105905 (2022)
S.M. Tabatabaeinejad, S. Zinatloo-Ajabshir, O. Amiri, M. Salavati-Niasari, Magnetic Lu2Cu2O5-based ceramic nanostructured materials fabricated by a simple and green approach for an effective photocatalytic degradation of organic contamination. RSC Adv. 11, 40100–40111 (2021)
A. Zonarsaghar, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Hydrothermal synthesis of CeVO4 nanostructures with different morphologies for electrochemical hydrogen storage. Ceram. Int. 47, 35248–35259 (2021)
A. Zonarsaghar, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Sonochemical synthesis of CeVO4 nanoparticles for electrochemical hydrogen storage. Int. J. Hydrog. Energy. 47, 5403–5417 (2022)
S. Zinatloo-Ajabshir, M.S. Morassaei, O. Amiri, M. Salavati-Niasari, L.K. Foong, Nd2Sn2O7 nanostructures: green synthesis and characterization using date palm extract, a potential electrochemical hydrogen storage material. Ceram. Int. 46, 17186–17196 (2020)
M. Ghodrati, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Zn3V3O8 nanostructures: facile hydrothermal/solvothermal synthesis, characterization, and electrochemical hydrogen storage. Ceram. Int. 46, 28894–28902 (2020)
S. Zinatloo-Ajabshir, M.S. Morassaei, M. Salavati-Niasari, Simple approach for the synthesis of Dy2Sn2O7 nanostructures as a hydrogen storage material from banana juice. J. Clean. Prod. 222, 103–110 (2019)
M.M. Lakourj, R.S. Norouzian, M. Esfandyar, Conducting nanocomposites of polypyrrole-co-polyindole doped with carboxylated CNT: Synthesis approach and anticorrosion/antibacterial/antioxidation property. J. Mater. Sci. Eng. B. 261, 114673–114688 (2020)
S.M. Ebrahim, Fabrication of Schottky diode based on Zn electrode and polyaniline doped with 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt. J. Polym. Res. 16, 481–487 (2009)
T.M. Abdel-Fattah, S. Ebrahim, M. Soliman, M. Hafez, Dye-sensitized solar cells based on polyaniline-single wall carbon nanotubes composite. ECS J. Solid State Sci. Technol. 2, M13–M16 (2013)
M. Shoeb, M. Mobin, M.A. Rauf, M. Owais, A.H. Naqvi, In vitro and in vivo antimicrobial evaluation of graphene-polyindole (Gr@PIn) nanocomposite against methicillin-resistant staphylococcus aureus pathogen. ACS Omega 3, 9431–9440 (2018)
A. Verma, R.B. Choudhary, Influence of CdS nanorods on the optoelectronic properties of 2-dimensional rGO decorated polyindole matrix, Mat. Sci. Semicon. Proc. 110, 104948 (2020)
M.T. Ramesan, Fabrication and characterization of conducting nanomaterials composed of copper sulfide and polyindole. Polym. Compos. 33(12), 2169–2176 (2012)
R.E. Palacios, K.J. Lee, A. Rival, T. Adachi, J.C. Bolinger, L. Fradkin, P.F. Barbara, Single conjugated polymer nanoparticle capacitors. Chem. Phys. 357(1–3), 21–27 (2009)
C.J. Verma, R.K. Pandey, R. Prakash, Insitu one step synthesis of Fe inserted octaethylporphyrin/polyindole: a multifunctional hybrid material with improved electrochemical and electrical properties. J. Mater. Sci. Eng. B. 227, 80–88 (2018)
M. Habib, M. Feteha, M. Soliman, A.A. Motagaly, S. El-Sheikh, S. Ebrahim, Effect of doped polyaniline/graphene oxide ratio as a hole transport layer on the performance of perovskite solar cell. J. Mater. Sci. 31, 18870–18882 (2020)
N.S. Wadatkar, S.A. Waghuley, Complex optical studies on conducting polyindole as-synthesized through chemical route. Egypt. J. Basic Appl. Sci. 2(1), 19–24 (2015)
B. Sari, N. Yavas, M. Makulogullari, O. Erol, H.I. Unal, Synthesis, electrorheology and creep behavior of polyindole/polyethylene composites. React. Funct. Polym. 69(11), 808–815 (2009)
N.S. Wadatkar, S.A. Waghuley, A novel studies on electrical behaviour of chemically synthesized conducting polyindole. Indian J. Phys. 92(12), 1551–1559 (2018)
K. Hasuksom, W. Prissanaroon-Ouajai, A. Sirivat, A highly responsive methanol sensor based on graphene oxide/polyindole composites. RSC Adv. 10(26), 15206–15220 (2020)
D.J. Bhagat, G.R. Dhokane, Novel photoluminescence and optical investigation of poly(vinylacetate)/polyindole composites synthesized via chromium chloride as oxidant. Appl. Surf. Sci. 351, 1140–1145 (2015)
H. Mudila, P. Prasher, M. Kumar, A. Kumar, M. Zaidi, A. Kumar, Critical analysis of polyindole and its composites in supercapacitor application. Mater. Renew. Sustain. Energy. 8, 1–19 (2019)
Q. Zhou, D. Zhu, X. Ma, J. Xu, W. Zhou, F. Zhao, High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv. 6(35), 29840–29847 (2016)
D. Bera, L. Qian, T.K. Tseng, P.H. Holloway, Quantum dots and their multimodal applications. Materials 3(4), 2260–2345 (2010)
M.S. Zaini, C. Ying, S.A. Alang Ahmad, A.R. Mohmad, M.A. Kamarudin, Quantum confinement effect and photoenhancement of photoluminescence of PbS and PbS/MnS quantum dots. Appl. Sci. 10(18), 6282–6289 (2020)
Y. Shirasaki, G.J. Supran, M.G. Bawendi, V. Bulović, Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 7(1), 13–20 (2013)
P.G. Luo, S. Sahu, S.T. Yang, S.K. Sonkar, J. Wang, H. Wang, Y.P. Sun, Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B. 1(16), 2116–2127 (2013)
S. Ebrahim, A. Shokry, M.M.A. Khalil, H. Ibrahim, M. Soliman, Polyaniline/Ag nanoparticles/graphene oxide nanocomposite fluorescent sensor for recognition of chromium (VI) ions. Sci. Rep. 10(1), 1–11 (2020)
A. Ramadan, M. Anas, S. Ebrahim, M. Soliman, A. Abou-Aly, Effect of Co-doped graphene quantum dots to polyaniline ratio on performance of supercapacitor. J. Mater. Sci. 31(9), 7247–7259 (2020)
U. Abd Rani, L.Y. Ng, C.Y. Ng, E. Mahmoudi, A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci. 278, 102124 (2020)
H. Heidarzadeh, A. Rostami, M. Dolatyari, Management of losses (thermalization-transmission) in the Si-QDs inside 3C–SiC to design an ultra-high-efficiency solar cell. Mater. Sci. Semicond. Process. 109, 104936 (2020)
Y. Chen, S. Li, L. Huang, D. Pan, Green and facile synthesis of water-soluble Cu–In–S/ZnS core/shell quantum dots. Inorg. Chem. 52(14), 7819–7821 (2013)
B. Zhang, Y. Wang, C. Yang, S. Hu, Y. Gao, Y. Zhang, K.T. Yong, The composition effect on the optical properties of aqueous synthesized Cu–In–S and Zn–Cu–In–S quantum dot nanocrystals. Phys. Chem. Chem. Phys. 17(38), 25133–25141 (2015)
M. Jiao, X. Huang, L. Ma, Y. Li, P. Zhang, X. Wei, M. Gao, Biocompatible off-stoichiometric copper indium sulfide quantum dots with tunable near-infrared emission via aqueous based synthesis. Chem Commun. 55(100), 15053–15056 (2019)
M. Arora, B. Kiran, S. Rani, A. Rani, B. Kaur, N. Mittal, Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 111, 811–815 (2008)
K.H. Vardhan, P.S. Kumar, R.C. Panda, A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J. Mol. Liq. 290, 111197–111218 (2019)
H. Singh, A. Bamrah, S.K. Bhardwaj, A. Deep, M. Khatri, K.H. Kim, N. Bhardwaj, Nanomaterial-based fluorescent sensors for the detection of lead ions. J. Hazard. Mater. 407, 124379 (2021)
M. Shehab, S. Ebrahim, M. Soliman, Graphene quantum dots prepared from glucose as optical sensor for glucose. J. Lumin. 184, 110–116 (2017)
R.M. Bouldin, A. Singh, M. Magaletta, S. Connor, J. Kumar, R. Nagarajan, Biocatalytic synthesis of fluorescent conjugated indole oligomers. Bioengineering 1(4), 246–259 (2014)
P. Chhattise, K. Handore, A. Horne, K. Mohite, A. Chaskar, S. Dallavalle, V. Chabukswar, Synthesis and characterization of Polyindole and its catalytic performance study as a heterogeneous catalyst. J. Chem. Sci. 128(3), 467–475 (2016)
K.N. Handore, S.V. Bhavsar, N. Pande, P.K. Chhattise, S.B. Sharma, S. Dallavalle, V.V. Chabukswar, Polyindole-ZnO nanocomposite: synthesis, characterization and heterogeneous catalyst for the 3, 4-dihydropyrimidinone synthesis under solvent-free conditions. Polym. Plast. Technol. Eng. 53(7), 734–741 (2014)
F. Zhang, X. He, P. Ma, Y. Sun, X. Wang, D. Song, Rapid aqueous synthesis of CuInS/ZnS quantum dots as sensor probe for alkaline phosphatase detection and targeted imaging in cancer cells. Talanta 189, 411–417 (2018)
R. Adel, S. Ebrahim, A. Shokry, M. Soliman, M. Khalil, Nanocomposite of CuInS/ZnS and nitrogen-doped graphene quantum dots for cholesterol sensing. ACS Omega 6(3), 2167–2176 (2021)
N.T. Vo, H.D. Ngo, D.L. Vu, A.P. Duong, Q.V. Lam, Conjugation of E. coli O157: H7 antibody to CdSe/ZnS quantum dots. J. Nanomater. (2015). https://doi.org/10.1155/2015/265315
M.T. Ramensan, Synthesis and characterization of magnetoelectric nanomaterial composed of Fe3O4 and polyindole. Adv. Polym. Technol. 32, 1–9 (2013)
R. Mishra, N.R. Nirala, R.K. Pandey, R.P. Ojha, R. Prakash, Homogenous dispersion of MoS2 nanosheets in polyindole matrix at air-water interface assisted by langmuir technique. Langmuir 33(47), 13572–13580 (2017)
J. El Nady, M. Ali, O.A. Kamel, S. Ebrahim, M. Soliman, Room temperature synthesis of aqueous ZnCuInS/ZnS quantum dots. J. Disper. Sci. Technol. 41(13), 1956–1962 (2020)
V. Ajeet, B.C. Ram, N. Debashish, M. Gobind, Structural analysis with augmented optoelectronic performance of polyindole–polypyrrole–cadmium sulfide nanohybrid: PIN/PPY/ CdS. J Inorg Organometall Polym Mater. 30(7), 2683–2691 (2020)
M. Patrycja, P. Michał, M. Wojciech, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 9(23), 6814–6817 (2018)
T. Anjitha, T. Anilkumar, G. Mathew, M.T. Ramesan, Zinc ferrite@ polyindole nanocomposites: synthesis, characterization and gas sensing applications. Polym. Compos. 40(7), 2802–2811 (2019)
M. Ali, J. El Nady, S. Ebrahim, M. Soliman, Structural and optical properties of upconversion CuInS/ZnS quantum dots. Opt. Mater. 86, 545–549 (2018)
M. Mou, Y. Wu, H. Zou, J. Dong, S. Wu, Z. Yan, S. Liao, The self-assembly of Cu-In-S quantum dots with aggregation-induced emission into 3D network triggered by cation and its application as a novel metal-enhanced fluorescent nanosensor for detecting Zn (II). Sens. Actuators B: Chem. 284, 265–270 (2019)
C.W. Chen, D.Y. Wu, Y.C. Chan, C.C. Lin, P.H. Chung, M. Hsiao, R.S. Liu, Evaluations of the chemical stability and cytotoxicity of CuInS2 and CuInS2/ZnS core/shell quantum dots. J. Phys. Chem. C. 119(5), 2852–2860 (2015)
L. Yang, A. Antanovich, A. Prudnikau, O.S. Taniya, K.V. Grzhegorzhevskii, P. Zelenovskiy, M. Artemyev, Highly luminescent Zn–Cu–In–S/ZnS core/gradient shell quantum dots prepared from indium sulfide by cation exchange for cell labeling and polymer composites. Nanotechnology 30(39), 395603 (2019)
W.S. Song, J.H. Kim, J.H. Lee, H.S. Lee, H.S. Jang, H. Yang, Utilization of LiSrPO4: Eu phosphor and Cu–In–S quantum dot for fabrication of high color rendering white light-emitting diode. Mater. Lett. 92, 325–329 (2013)
K. Ashish, P. Avinash, P. Rajiv, Electro-oxidation of formic acid using polyindole-SnO2 nanocomposite. Catal. Sci. Technol. 2(12), 2533–2538 (2012)
P. Dubey, A. Kumar, R. Prakash, Non-covalent functionalization of graphene oxide by polyindole and subsequent incorporation of Ag nanoparticles for electrochemical applications. Appl. Surf. Sci. 355, 262–267 (2015)
R. Yan, W. Zhang, W. Wu, X. Dong, Q. Wang, J. Fan, Optical spectroscopy reveals transition of CuInS2/ZnS to CuxZn1−xInS2/ZnS: Cu alloyed quantum dots with resultant double-defect luminescence. APL Mater. 4, 126101–126109 (2016)
D.F. Fang, Z.M. Zhang, Z.P. Wang, Z.J. Ding, Study of photoluminescence of CdS/ZnS core/shell quantum dots. Phys. Procedia. 32, 920–925 (2012)
S. Rosen, O. Schwartz, D. Oron, Transient fluorescence of the off state in blinking CdSe/CdS/ZnS semiconductor nanocrystals is not governed by auger recombination. Phys. Rev. Lett. 104, 157404 (2010)
N. Mondal, A. De, A. Samanta, All-inorganic perovskite nanocrystal assisted extraction of hot electrons and biexcitons from photoexcited CdTe quantum dots. Nanoscale 10(2), 639–645 (2018)
M. Aliofkhazraei, N. Ali, W.I. Milne, C.S. Ozkan, S. Mitura, J.L. Gervasoni, Graphene science handbook: nanostructure and atomic arrangement (CRC Press, Boca Raton, 2016)
R. Kubrin, W. Bauhofer, Influence of polymeric additives on morphology and performance of Y2O3: Eu phosphor synthesized by flame-assisted spray pyrolysis. J. Lumin. 129, 1060–1066 (2009)
S.J. Lim, M.U. Zahid, P. Le, L. Ma, D. Entenberg, A.S. Harney, J. Condeelis, A.M. Smith, Brightness-equalized quantum dots. Nat. Commun. 6, 8210 (2015)
N. Joffin, J. Dexpert-Ghys, M. Verelst, G. Baret, A. Garcia, The influence of microstructure on luminescent properties of Y2O3: Eu prepared by spray pyrolysis. J. Lumin. 113, 249–257 (2005)
S.J. Flora, M. Mittal, A. Mehta, Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 128, 501–523 (2008)
J. Aaseth, G. Crisponi, O. Andersen, Chelation therapy in the treatment of metal intoxication (Academic Press, New York, 2016)
S.J. Flora, V. Pachauri, Chelation in metal intoxication. Int. J. Environ. Res. Public Health. 7(7), 2745–2788 (2010)
M.E. Sears, Chelation: harnessing and enhancing heavy metal detoxification. Sci. World J. 213, 219840 (2013)
X. Han, F. Yu, J. Lei, J. Zhu, H. Fu, J. Hu, X.L. Yang, Pb2+ Responsive Cu–In–Zn–S quantum dots with low cytotoxicity. Front. Chem. (2022). https://doi.org/10.3389/fchem.2022.821392
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkony, Y., Ali, M., Ebrahim, S. et al. High Photoluminescence Polyindole/CuInS Quantum Dots for Pb Ions Sensor. J Inorg Organomet Polym 32, 3106–3116 (2022). https://doi.org/10.1007/s10904-022-02300-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02300-5