Abstract
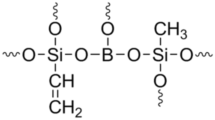
Polycondensation of boric acid (BA) with mixtures of phenyltriethoxysilane (PTEOS) and vinyltriethoxysilane (VTEOS) and with mixtures of phenyltrimethoxysilane (PTMOS) and vinyltriethoxysilane (VTEOS) in diglyme at 83–87 °C using HCl as catalyst resulted in vinyl-functionalized borosiloxane oligomers. Effect of variation of PTEOS:VTEOS and PTMOS:VTEOS ratio (keeping BA:alkoxysilanes ratio constant) and the effect of variation of BA:alkoxysilane (BA:PTEOS:VTEOS and BA:PTMOS:VTEOS) ratio on the solubility, thermal stability and ceramic residue were studied. The oligomers obtained were characterized by FTIR, GPC, pyrolysis GC and TGA. For PTEOS + VTEOS system all the oligomers were soluble in the reaction medium, but after removal of alcohol (byproduct) and diglyme (solvent), the oligomers obtained in the solid form were insoluble in common organic solvents. Unlike the PTEOS-based system, for PTMOS-based system the oligomers synthesized from monomer feed ratios (BA:PTMOS:VTEOS) 1:1:1 and 1:1.67:0.33 even after the removal of ethanol and diglyme are soluble in tetrahydrofuran, dioxane and diglyme. These two soluble oligomers show bimodal molecular weight distribution with \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 3,650 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1860 for 1:1:1 mol ratio, and \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 2,540 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1700 for 1:1.67:0.33 mol ratio. 29Si-NMR spectra of the soluble oligomers show peaks at − 69 and − 78 ppm which are attributed to T2 and T3 structures respectively. Thermogravimetric analysis of vinyl-functionalized borosiloxane oligomers from PTEOS and PTMOS indicates that the ceramic residue of the oligomers in argon atmosphere at 900 °C varies from 68 to 89% depending on the monomer feed ratio of BA to organoalkoxysilane ratio and phenyltrialkoxysilane to VTEOS ratio. With the increase in concentration of VTEOS in the monomer feed the thermal stability as well as the ceramic residue of the oligomers increase and a reverse trend is observed for variation of BA concentration. Pyrolysis of the oligomers produce C2, C3 hydrocarbons and benzene as the main pyrolysis products. Ceramic conversion of a typical oligomer was carried out at 900 °C, 1500 °C and 1650 °C in argon atmosphere. The ceramics obtained at 900 °C and 1500 °C are amorphous SiBOC which transform to β-SiC at 1650 °C.














Similar content being viewed by others
References
J.E. Mark, H.R. Allcock, R. West, Inorganic Polymers, 2nd edn. (Oxford University Press, New York, 2005).
A.H. McKinney, Inorganic-Organic Hybrid Polymers for High-Temperature Applications, Technical Briefs, vol. 42, issue 1 (Naval Research Laboratory, Washington DC, 2018)
K.J.D. MacKenzie, Innovative Applications of Inorganic Polymers (Geopolymers), Chapter 28 in Handbook of Alkali-Activated Cements, Mortars and Concretes (Elsevier, Amsterdam, 2015), pp. 777–805
K.A. Williams, A.J. Boydston, C.W. Bielawski, Main-chain organometallic polymers: synthetic strategies, applications, and perspectives. Chem. Soc. Rev. 36, 729–744 (2007)
P. Colombo, G. Mera, R. Riedel, G.D. Soraru, Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 93, 1805–1837 (2010)
S. Packirisamy, Decaborane(14)-based polymers. Prog. Polym. Sci. 21, 707–773 (1996)
S. Packirisamy, D. Schwam, M.H. Litt, Atomic oxygen resistant coatings for low earth orbit space structures. J. Mater. Sci. 30, 308–320 (1995)
D. Devapal, S. Packirisamy, R.M. Korulla, K.N. Ninan, Atomic oxygen resistant coating from poly (tetramethyldisilylene-co-styrene). J. Appl. Polym. Sci. 94(6), 2368–2375 (2004)
D. Devapal, S. Packirisamy, C.P.R. Nair, K.N. Ninan, Phosphazene-based polymers as atomic oxygen resistant materials. J. Mater. Sci. 41, 5764–5766 (2006)
P. Innocenzi, B. Lebeau, Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 15, 3821–3831 (2005)
S. Packirisamy, K.J. Sreejith D. Devapal, B. Swaminathan, Polymer-derived ceramics and their space applications, in Handbook of Advanced Ceramics and Composites. ed. by Y. Mahajan, J. Roy (Springer Nature, Cham, 2000), pp. 975–1080
S. Fu, M. Zhu, Y. Zhu, Organosilicon polymer-derived ceramics: an overview. J. Adv. Ceram. 8, 457–478 (2019)
A. Xia, J. Yin, X. Chen, X. Liu, Z. Huang, Polymer-derived si-based ceramics: recent developments and perspectives. Crystals 10(9), 824 (2020)
A. Viard, D. Fonblanc, D. Lopez-Ferber, M. Schmidt, A. Lale, C. Durif, S. Bernard, Polymer derived Si-B-C-N ceramics: 30 years of research. Adv. Eng. Mater. 20, 1800360 (2018)
E. Ionescu, S. Bernard, R. Lucas, P. Kroll, S. Ushakov, A. Navrotsky, R. Riedel, Polymer-derived Ultra-High Temperature Ceramics (UHTCs) and related materials. Adv. Eng. Mater. 21, 1900269 (2019)
G.D. Soraru, E. Dallapiccola, G. Dandrea, Mechanical characterization of sol–gel-derived silicon oxycarbide glasses. J. Am. Ceram. Soc. 79, 2074–2080 (1996)
J. Parmentier, G.D. Soraru, F. Banonneau, Influence of the microstructure on the high temperature behavior of gel-derived SiOC glasses. J. Eur. Ceram. Soc. 21, 101–108 (2001)
G.D. Soraru, L. Pederiva, J. Latournerie, R. Raj, Pyrolysis kinetics for the conversion of a polymer into an amorphous silicon oxycarbide ceramic. J. Am. Ceram. Soc. 85, 2181–2187 (2002)
A. Saha, R. Raj, Crystallization maps for SiCO amorphous ceramics. J. Am. Ceram. Soc. 90, 578–583 (2007)
C. Stabler, E. Ionescu, M. Graczyk-Zajac, I. Gonzalo-Juan, R. Riedel, Silicon oxycarbide glasses and glass ceramics: “all-rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 101, 4817–4856 (2018)
B.V. Manoj Kumar, Y.-W. Kim, Processing of polysiloxane-derived porous ceramics: a review. Sci. Technol. Adv. Mater. 11, 044303 (2010)
K. Lu, D. Erb, Polymer derived silicon oxycarbide-based coatings. Int. Mater. Rev. 63, 139–161 (2017)
G.D. Soraru, F. Babonneau, S. Maurina, J. Vicens, Sol–gel synthesis of SiBOC glasses. J. Non-Cryst. Solids 224, 173–183 (1998)
G.D. Soraru, N. Dallabona, C. Gervais, F. Babonneau, Organically modified SiO2-B2O3 gels displaying a high content of borosiloxane (B-O-Si) bonds. Chem. Mater. 11, 910–919 (1999)
G. Ambadas, S. Packirisamy, K.N. Ninan, Synthesis, characterization and thermal properties of boron and silicon containing preceramic oligomers. J. Mater. Sci. Lett. 21, 1003–1005 (2002)
C. Gervais, F. Babonneau, N. Dallabonna, G.D. Sorarù, Sol–gel-derived silicon-boron oxycarbide glasses containing mixed silicon oxycarbide (SiCxO4−x) and boron oxycarbide (BCyO3−y) units. J. Am. Ceram. Soc. 84, 2160–2164 (2004)
M.A. Schiavon, C. Gervais, F. Babonneau, G.D. Soraru, Crystallization behavior of novel silicon boron oxycarbide glasses. J. Am. Ceram. Soc. 87, 203–208 (2004)
M.A. Schiavon, N.A. Armelin, I. Yoshida, Novel poly(borosiloxane) precursors to amorphous SiBCO ceramics. Mater. Chem. Phys. 112, 1047–1054 (2008)
D. Devapal, S. Packirisamy, K.J. Sreejith, P.V. Ravindran, B.K. George, Synthesis, characterization and ceramic conversion studies of borosiloxane oligomers from phenyltrialkoxysilanes. J. Inorg. Organomet. Polym. Mater. 20, 666–674 (2010)
K.J. Sreejith, P.V. Prabhakaran, K.P. Laly, R. Dimple, S. Packirisamy, Vinyl-functionalized poly(borosiloxane) as precursor for SiC/SiBOC nanocomposite. Ceram. Int. 42, 15285–15293 (2016)
D. Devapal, K.J. Sreejith, B. Swaminathan, S. Chinthalapalli, S. Bhuvaneswari, S. Packirisamy, Influence of heat treatment temperature on the microstructure evolution of poly(vinylborosiloxane) derived ceramics. J. Inorg. Organomet. Polym. Mater. 30, 2224–2233 (2020)
S. Rubinsztajn, New facile process for synthesis of borosiloxane resins. J. Inorg. Organomet. Polym. Mater. 24, 1092–1095 (2014)
D. Devapal, Studies on inorganic and organometallic polymers. PhD thesis, Mahatma Gandhi University, Kottayam (2007)
P.V. Prabhakaran, Studies on non-oxide ceramics derived from polymers and their applications. PhD thesis, University of Kerala, Thiruvananthapuram, 2008
K. J. Sreejith, Polymer derived ceramics and their high temperature applications. PhD thesis, University of Kerala, Thiruvananthapuram, 2010
J.A. Perry, Introduction to Analytical Gas Chromatography: History, Principles and Practice (Marcel Dekker, New York, 1981).
G.D. Soraru, F. Babonneau, C. Gervais, N. Dallabona, Hybrid RSiO1.5/B2O3 gels from modified silicon alkoxides and boric acid. J. Sol–Gel. Sci. Technol. 18, 11–19 (2000)
Acknowledgements
The authors thank the authorities of VSSC for granting permission to publish this work. Help received from the members of the Analytical and Spectroscopy Division for the thermal, chemical and spectral analysis of the samples is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest with respect to the data presented in the paper. There is also transparency of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devapal, D., Varughese, G., Radhakrishnan, T.S. et al. Studies on Borosiloxane Oligomers from Mixtures of Vinyltriethoxysilane and Phenyltrialkoxysilanes. J Inorg Organomet Polym 31, 2672–2681 (2021). https://doi.org/10.1007/s10904-021-01964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01964-9