Abstract
The aim of the research was to obtain biopolymers based on wheat gluten, whey protein concentrate (WPC) and montmorillonite (MON). Ternary biopolymers were formed as heat-induced gels and they were hardened by water evaporation. Adding 7 % of MON and 5 % WPC to gluten caused ca. four times higher values of storage modulus. Increased moduli values with adding MON and WPC to gluten were probably caused by reinforcing effect of MON, which decreased mobility of gluten chains and possible interactions between gluten and whey proteins by disulphide interchange. Increase in gluten protein concentration and addition of WPC and MON caused increase in viscosity measured by dissipation of ultrasound vibrations. Evaporation of water from the gels formed very hard material with high puncture force values. Obtained gels were very plastic and it was easy to form any type of shapes. They could be used to produce biodegradable pottery (e.g. flowerpots) with the mechanical properties similar to non-degradable clay products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The annual growth of the global production of plastics is approximately 5 %. An increase in non-degradable plastic waste have caused significant environmental concerns about natural sources for polymers. Petroleum-based synthetic plastics are unable to degrade in landfill or a compost [1]. Biodegradable materials obtained from agricultural resources such as proteins, starch or other carbohydrates have attracted much attention by biopolymers manufacturers. It is predicted, that production capacity of biopolymers will triple from 3.5 million tonnes in 2011 to 12 million tonnes in 2020 [2]. Natural polymer designers’ aim is the development of a product which can mimic the microstructure and function of a synthetic polymer. Completely natural composites and polymers are created, or synthetic polymers’ microstructure is reinforced by bioingredients acting as fillers or active matrix constituents [3]. The natural polymers can not only reduce soil degradation, but also increase its fertility [4]. Lately biopolymers have been used as pesticide carriers. Besides their ecofriendly biodegradability and non-ecotoxicity, it is easier than for plastics to design controlled release formulations [5]. The encapsulation of a chemical fertilizer in a biopolymer matrix could decrease environmental pollution of ground water [6]. Konjak glukomannan/poly(vinyl alcohol)/Na+ Rectorite biodegradable films can be used as drug delivery matrix [7]. Among all agricultural sources, proteins (animal: myofibrillar proteins. milk proteins, collagen, gelatin, bovine serum albumin, silk protein, and vegetable: gluten, zein, soy protein) are the most interesting ingredient of natural biopolymers [8]. Bioplastics from protein sources have been used for various applications including matrices for enzyme immobilization and active ingredients controlled-release, active packaging technology, natural fiber reinforcements, nanotechnology, tissue engineering, wound dressings and membrane filters [9]. Plant proteins are very attractive for bioplastics production because of their physicochemical properties, abundant resources and low cost. Wheat gluten (WG) being a by-product of the wheat starch and bioethanol industries is available at a price around 1.3 $/kg [1]. Wheat gluten is a general term for proteins of wheat flour which is composed of a mixture of globular proteins: gliadins and glutenins [10]. Gliadins are monomeric proteins and glutenins a macropolymer. Upon heating gluten proteins undergo disulfide interchange leading to 3-dimensional macromolecule [11]. Wheat gluten is fully biodegradable within 50 days in soil without releasing toxic products [3]. Wheat gluten biomaterials can be manufactured using several processing methods, including extrusion, compression molding and solution casting. One possibility for expanding the potential areas of application is to reinforce the microstructure by bio-fibers such as: flax, jute, coconut and nettles [12]. Recent studies have reported the use of wheat gluten reinforced with hemp fibers to make biocomposites with improved mechanical properties [13]. The elasticity and cohesiveness of gluten is used in development of strong edible films. The gluten films have good barrier properties for oxygen and carbon dioxide and are highly permeable to water [14, 15]. Montmorillonite (MON), displaying a smectite-type structure, is one of the most commonly used layered silicates because of its quite low cost. It is widely used for various applications such as barrier clays, cement, catalysts, ceramics, cosmetics, drilling fluid, adhesives, food additives, paint or paper ingredients [16]. Films containing mixtures of biopolymers and montmorillonite possess improved mechanical strength, better water vapor and gas barrier characteristics, and enhanced heat and solvent resistance [17, 18]. Adding of unmodified MON did not influence the final biodegradation level of gluten based biopolymers [1]. Zárate-Ramíreza et al. [2] investigated the influence of adding different polysaccharides (locust bean gum, LBG; methyl cellulose, MC; and carboxymethyl cellulose, CMC) to gluten-based biodegradable polymeric materials. Results showed, that a moderate enhancement of the network structure may be achieved by adding polysaccharide. Türea et al. [19] showed that both WG and MON increased the water absorbency of paperboards. Delivery system based on a WG matrix reinforced by 5 % MON was clearly efficient to retain and protect an antimicrobial active agent such as carvacrol during the processing stage [20]. Adding of MON to WG objects produced by injection molding improved processability, plate homogeneity and tensile stiffness of the products [21]. Generally, protein based biodegradable materials are manufactured by two technological processes: a wet process technology based on dispersion of proteins and a dry process based on the thermoplastic properties of proteins under low-moisture conditions [14]. Whey proteins are very popular functional ingredient in foods. Their texture can be shaped by gelation process, caused by heating of whey protein solution above the denaturation temperature [22]. In our last research mixed whey protein/montmorillonite biopolymers were formed as heat-induced gels hardened by water evaporation. Very strong biopolymers were obtained, but the aim of the presented research is to produce even harder material which would mimic ceramic-like materials like in e.g. pottery production [23]. In our research a unique method of gels analysis has been used: ultrasound viscosity measurements. In ultrasound viscosity testing, a magnetostrictive probe is moved by the phenomenon of magnetostriction, i.e., the deformation of ferromagnetic materials by electric current, which produces free vibration. Vibration creates ultrasound waves which are damped by the tested material. Ultrasound measurements similarly to oscillatory rheological method, uses small strains and does not cause destruction of the sample. Ultrasound viscometers display results as the product of viscosity x density. Density of the gels is close to 1 and the results can be approximately treated as viscosity values [24].
The aim of the research is to obtain a material similar to ceramic material to use it to produce biodegradable pottery (e.g. flowerpots). Clay flowerpots, although not encumbering environment, are a burden for soil as they are practically non-degradable.
2 Experimental
2.1 Materials
Wheat gluten (WG) powder was supplied by Massive (Czechowice-Dziedzice, Poland). According to the provider, the powder consisted of 84.1 % protein, 7.8 % starch, 1.51 % fat, 0.69 % ash and 5.5 % water. Whey protein concentrate (WPC) with 80.0 % protein and 3.4 % ash was purchased from SM Spomlek (Radzyń Podlaski, Poland). Protein concentration was determined by the Kjeldahl procedure [25]. Minerals content was provided by the producer. Montmorillonite (MON) with surface area 220–270 m2/g based on the product specification (Sigma-Aldrich, Poznań, Poland) was used.
2.2 Samples Preparation
Wheat gluten was preliminary mixed with whey protein concentrate and montmorillonite in plastic bags. The powder was hydrated in distilled water to obtain dispersions with 7 % (w/w) protein from WPC, 5 % (w/w) MON and gluten in the concentration: 10, 15, 20, 25 or 30 % (w/w). WPC protein and MON concentration have been chosen based on previous research results [23]. For hydrating a magnetic plate was used. Dispersions (50 cm3) were heated in water bath in 150 cm3 beakers for 30 min at 80 °C. After heating the samples were immediately cooled down with cold tap water for 10 min. Obtained biopolymers in the form of cylinders (ca. 50 mm in diameter and 25 mm high) were stored at 7 °C for 18 h. All the samples were equilibrated at 21 °C for 3 h and subjected to evaluations of their physicochemical properties. To obtain dry biopolymer material, gel cylinders (ca. 50 mm in diameter and ca. 3 mm high) were dried in 100 mm dimeter Petri dishes in a thermostatic cabinet ST3/3 (Pol-Eko-Aparatura, Wodzisław Śląski, Poland) for 72 h at 40 °C. Every 12 h the sample was turned over. The final water content in the sample was in the range 16.3–17.9 % and it was measured by drying the samples at 105 °C to the constant mass.
2.3 Dynamic Oscillatory Measurements
Dynamic rheological measurements were performed using RS300 (ThermoHaake, Karlsruhe, Germany) rheometer with a serrated parallel steel plate geometry (35 mm diameter, 2 mm gap size) to limit the potentiality of sliding effects. Samples were cut by a surgical scalpel (35 mm in diameter and 2.5 mm high) and analyzed by frequency sweeps at 0.1-10 Hz range in linear viscoelastic region (at 0.01 strain evaluated previously by strain sweeps). All the measurements were performed at the temperature 21 °C.
2.4 Ultrasound Viscosity Measurements
An ultrasound viscometer Unipan type 505 (UNIPAN, Warsaw, Poland) probe was immersed into the heated samples before drying. The value of viscosity x density in mPas x g/cm3 was measured. Each result has been shown as an average of six measurements.
2.5 Puncture Tests
Puncture test was performed on dried biopolymer samples using a P/2 N needle probe. The samples were analyzed on a 15-mm thick metal plate with a 10-mm diameter hole in the center and a crosshead speed of 1 mm s−1. The upper surface of a dried sample was fixed by a 3 mm steel plate with a 10-mm diameter hole in the center to prevent the warping of the sample. The thickness of the samples was 2.0 mm at the test area and it was measured using a micrometer with an accuracy of 0.01 mm. Three measurements were carried out to obtain each result.
2.6 Scanning Electron Microscopy (SEM)
Dried biopolymers were fixed by immersion in 2.5 % glutaraldehyde solution in 0.1 M sodium cacodylate buffer. The samples were dehydrated in serial dilutions of ethanol and acetone and dried at the critical point in liquid carbon dioxide. Preparations were coated with gold using a vacuum evaporator EMITECH K550x (Emitech, Ashford, United Kingdom). Samples were viewed and photographed using a scanning electron microscope JEOL JXA-8230 (Tokyo, Japan). The elemental mapping of the samples was performed by detecting the X-rays emitted on excitation by a focused electron beam.
2.7 Polarizing Optical Microscopy
Wheat gluten powder was observed using a polarizing optical microscope Eclipse E600 Pol (Nikon, Tokyo, Japan).
2.8 Statistical Analysis
Statistical analysis of results (standard deviation, analysis of variance) was performed using the statistical program STATISTICA 5.0 PL (StatSoft Polska, Warsaw, Poland). The significance of differences between means was determined using the Tukey’s test at confidence level of P ≤ 0.05 based on the least significant difference.
3 Results and Discussion
3.1 Rheological Properties
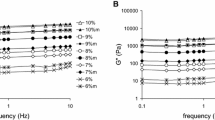
Figure 1 shows frequency sweep and changes in storage modulus G′ and loss modulus G″ of the ternary biopolymers obtained at different gluten concentration and compared with gluten gels. Increase in gluten concentration caused an increase in the moduli value. Gluten is, in the investigated ternary biopolymer, the main constituent influencing rheological properties. Increase in gluten density results in more dense microstructure caused by the chemical cross-linking of glutenins and gliadins. Lutz et al. [26] identified cystine peptides responsible for a “head-to-tail” disulfide bonds between high molecular weight glutenin subunits. The researchers have proven experimentally this type of cross-link, which had been previously postulated as an integral part of gluten gel formation. As water and powdered gluten are mixed the hydrated proteins are brought together to interact with each other. At the hydration process gluten proteins absorb about twice their weight in water [27]. Increased storage modulus with increased gluten concentration shows development of stronger and stronger elastic gel matrix.
Adding 7 % of MON and 5 % WPC to gluten caused a different increase in storage modulus depending on gluten concentration (from 3.5 to 7.8 times). Similarly adding of MON to polyimide matrix increased storage modulus of obtained composites [28]. All investigated gels behaved as weak physical gels as storage moduli values were several times higher than loss moduli and increase in frequency caused an increase in the moduli values. Increased frequency caused that the material had less time for relaxation and it acted as more elastic with higher values of storage moduli. Investigated material was characterized as physical gels, because tangent (δ) at all measured frequencies was in a range 0.1–1.0 (chart not shown). Materials with tangent (δ) close to 1 are concentrated biopolymers and a true chemical gel has tangent (δ) bellow 0.1 [29]. Increased moduli values with MON and WPC added were probably caused by reinforcing effect of MON, which decreased mobility of gluten chains and possible interactions between gluten and whey proteins [30]. Gluten proteins can undergo disulphide interchange upon heating, which leads to the formation of a 3-dimensional matrix. Disulphide interchange between gluten and whey proteins was probably responsible for formation strong mixed biopolymer with high storage modulus (Fig. 1) [1]. Woerdeman et al. [31] showed that modification of wheat gluten with a dithiothreitol can toughen the otherwise brittle wheat gluten material. Dithiothreitol is often used to break the disulfide bonds that link the various glutenin subunits to each other. Converting existing intramolecular disulfide bonds to free sulfhydryl groups is a preliminary requirement for success of an intermolecular disulfide bonds between gluten and whey proteins.
Figure 2 presents changes in the product of viscosity × density obtained by an ultrasound viscometer. Increase in gluten concentration caused increase in the viscosity (Fig. 2). Relationship between gluten concentration and ultrasound viscosity has been fitted to exponential curve (R2 0.92–0.98). For ternary biopolymers increased gluten concentration resulted in faster increase in ultrasound viscosity than for gluten gels themselves. It was clearly seen by larger exponent value in exponential curve formula for ternary biopolymers than for gluten gels. There is strong linear correlation between ultrasound viscosity values and G′ and G″ moduli values (charts not shown). It can be expressed as: ultrasound viscosity = 0.0278 G′ + 148.56 (R2 = 0.922) and ultrasound viscosity = 0.0859 G″ + 150.06 (R2 = 0.927).
Effect of gluten protein concentration on wheat gluten (G) (open) and wheat gluten/whey protein concentrate (WPC) (7 % w/w protein)/montmorillonite (MON) (5 % w/w) gels dynamic viscosity × density measured using ultrasound viscometer (differences between means marked with different letters are statistically significant P < 0.05)
3.2 Puncture Test
Figure 3 presents puncture force test for a sample of dried ternary biopolymer obtained at 25 % gluten. Two peaks are present on distance/force curve caused by a surface crack (the first one) and destruction of the sample. The breakthrough of the ternary dried biopolymer revealed cracks at the surface, what would support that the first peak was created as the biopolymer surface cracked. Increase in gluten concentration caused an increase in puncture force (Fig. 4). The maximum value of puncture force for 2 mm thick material punctured with 2 mm diameter needle was 187 N, which is much higher than the hardness of the WPC/MON biopolymer obtained in the previous research (80 N) [23]. Addition of MON and WPC to gluten reinforced the microstructure. Türe et al. [18] reported that the addition of MON into wheat gluten lead to improvements in the mechanical, barrier and thermal properties. Introducing the silica particles in the wheat gluten matrix led to better physical properties, as measured by 3-point bending [32]. In other research gluten microstructure was reinforced with unidirectional basalt fibers in a compression molding operation [33]. The WG/modified coconut fiber composite provided an 80 % increase in strength, and showed superior fiber–matrix interfacial adhesion [34]. Wheat gluten/montmorillonite films were prepared by a thermomechanical process. It consisted of mixing the components in a two-blade, counter-rotating device and then thermoforming of the obtaining dough. The introduction of MMT (up to 5 wt%) into gluten films allowed the achievement of mechanical properties that were not possible by just the variation of the glycerol content and the processing temperature [11]. To realize how strong was the dried ternary biopolymer we compared it with the hardness of bovine claw horn, which was evaluated by the same method. The hardness of the horn was ca. 25 N [35] and the hardness of the investigated biopolymer was ca. 187 N. It clearly shows, that the hardness of ternary biopolymer was 7.5 times harder than bovine claw horn. Undried biopolymer was soft and it was possible to form any shape. Drying caused formation of very strong material. Sharma and Luzinov [36] observed that ageing (water evaporation) during storage of protein biopolymers caused an increase in the mechanical properties of strength and stiffness.
3.3 Microstructure
Figure 5 presents microstructure of the ternary biopolymer surface obtained at 25 % gluten concentration. Smooth and compact microstructure is in agreement with strong mechanical properties of the investigated material. Figure 6 shows scanning electron microscopy of the ternary biopolymer surface with elements mapping. This method is used to visualize the regions with higher concentration of a specific element. All elements in the periodic table with an atomic number higher than oxygen can be identified in the element mapping. This is a qualitative method showing the distribution of elements in the sample. For ternary biopolymers the regions with higher concentration of protein (gluten and WPC) were mapped for carbon (Fig. 6a) and the regions with higher concentration of MON were mapped for silicon (Fig. 6b). To prove that the regions rich in proteins were poor in MON, a negative photo from carbon mapping was compared with a positive photo for silicon mapping (Fig. 6c, d). Figures show, that proteins created a biopolymer matrix with montmorillonite immersed into it and forming oval agglomerates. Previously good dispersion of organosilica domains into the organic polymer matrix has been shown [37]. Confocal laser scanning microscopy showed MON clay clusters dispersed in wheat gluten protein matrix [38]. Gluten and WPC mixed polymers can occupy the interlayer space of the clay causing a variation of the distance of this space. Extensive structural studies, involving X-ray scattering (WAXS and SAXS) and electron microscopy (TEM) techniques, revealed that adsorption of proteins causes MON to exfoliate, in a similar manner as in exfoliated polymer-layered silicate nanocomposites [39]. There is probably a synergistic adsorption of proteins on the surface of the clay. Future research is needed using atomic force microscopy, transmission electron microscopy, small-angle X-ray scattering and diffusing wave spectroscopy to explain how gluten/WPC protein polymer chains are incorporated in the clay structure.
Scanning electron microscopy showed a presence of fibers in the biopolymer matrix (Fig. 7a). The diameter of the fibers was about 7 μm. Similar fibers were observed by fluorescence microscopy method in gluten powder used for obtaining the biopolymers (Fig. 7b). Small size of the fibers and morphology of wheat grain suggest that the fibers could be the hairs of wheat grain brush. They can cause uneven mechanical properties within the biopolymer as observed by Wretfors et al. [12] for hemp fibers.
4 Conclusions
Hardening of mixed wheat gluten/whey protein concentrate/montmorillonite gels by water evaporation is a good method to obtain very strong biopolymers. Increased moduli values with adding MON and WPC to gluten were probably caused by reinforcing effect of MON, which decreased mobility of gluten chains and possible interactions between gluten and whey proteins by disulphide interchange. Obtained gels were very plastic and it was easy to form any type of shapes. They can be used to produce biodegradable pottery (e.g. flowerpots) with the mechanical properties similar to non-degradable clay products.
References
A. Chevillard, H. Angellier-Coussy, B. Cuq, V. Guillard, G. César, N. Gontard, E. Gastaldi, Polym. Degrad. Stab. 96, 2088 (2011)
L.S. Zárate-Ramíreza, A. Romerob, C. Bengoecheab, P. Partalc, A. Guerrerob, Carbohydr. Polym. 112, 24 (2011)
N. Patni, P. Yadava, A. Agarwal, V. Maroo, Rev. Chem. Eng. 30, 421 (2014)
P. Gupta, K.K. Nabak, Polym. Eng. Sci. 55, 485 (2015)
A. Chevillard, H. Angellier-Coussy, V. Guillard, N. Gontard, E. Gastaldi, Polym. Degrad. Stab. 97, 2060 (2012)
D. Gómez-Martínez, P. Partal, I. Martínez, C. Gallegos, Ind. Crops Prod. 43, 704 (2013)
L. Yang, X. Ma, N. Guo, Y. Zhang, J. Inorg. Organomet. Polym. 23, 1459 (2013)
I. Martínez, P. Partal, M. García-Morales, A. Guerrero, C. Gallegos, J. Food Eng. 117, 247 (2013)
D. Gómez-Martínez, P. Partal, I. Martínez, C. Gallegos, Bioresour. Technol. 100, 1828 (2009)
H. Wieser, Food Microbiol. 24, 115 (2007)
H. Angellier-Coussy, S. Torres-Giner, M.H. Morel, N. Gontard, E. Gastaldi, J. Appl. Polym. Sci. 107, 487 (2008)
C. Wretfors, S.W. Cho, M.S. Hedenqvist, S. Marttila, S. Nimmermark, E. Johansson, J. Polym. Environ. 17, 259 (2009)
F. Muneer, E. Johansson, M.S. Hedenqvist, M. Gällstedt, W.R. Newson, Bio. Res. 9, 5246 (2014)
D. Jagadeesh, D.J.P. Reddy, A.V. Rajulu, J. Polym. Environ. 19, 248 (2011)
I. Olabarrieta, M. Gällstedt, I. Ispizua, J.R. Sarasua, M.S. Hedenqvist, J. Agric. Food Chem. 54, 1283 (2006)
M. Hayati-Ashtiani, Part. Sci. Technol. 30, 553 (2012)
M.C. Villaman Dieguez, F.M. Pelissari, P.J. Amaral Sobral, F.C. Menegalli, Food Sci. Technol. 61, 70 (2015)
H. Türe, T.O.J. Blomfeldt, M. Gällstedt, M.S. Hedenqvist, J. Polym. Environ. 20, 1038 (2012)
H. Türe, M. Gällstedt, E. Johansson, M.S. Hedenqvist, Ind. Crops Prod. 51, 1 (2013)
E. Mascheroni, P. Chalier, N. Gontard, E. Gastaldi, Food Hydrocol. 24, 406 (2010)
S.W. Choa, M. Gallstedt, E. Johansson, M.S. Hedenqvist, Int. J. Biol. Macromol. 48, 146 (2011)
M. Tomczyńska-Mleko, Milchwissenschaft 67, 443 (2012)
M. Kawecka-Radomska, M. Tomczyńska-Mleko, M. Wesołowska-Trojanowska, K. Kowalczyk, M. Chrząstek, S. Mleko, J. Polym. Environ. 23, 534 (2015)
M. Tomczyńska-Mleko, L. Ozimek, J. Food Process Eng 36, 572 (2013)
AOAC, Official Methods of Analysis, vol. 52 (AOAC, Arlington, 1995), p. 960
E. Lutz, H. Wieser, P. Koehler, J. Agric. Food Chem. 60, 3708 (2012)
H. Xin-Zhong, S. Ya-Ning, J. Sci. Food Agric. 87, 1778 (2007)
J.O. Iroh, J. Longun, J. Inorg. Organomet. Polym. 22, 653 (2012)
M. Dolz, M.J. Hernandez, J. Delegido, J. Appl. Polym. Sci. 102, 897 (2006)
K. Fukushima, A. Rasyida, MCh. Yang, J. Polym. Res. 20, 302 (2013)
D.L. Woerdeman, W.S. Veraverbeke, R.S. Parnas, D. Johnson, J.A. Delcour, I. Verpoest, C.J.G. Plummer, Biomacromolecules 5, 1262 (2004)
S. Hemsri, A.D. Asandei, K. Grieco, R.S. Parnas, Compos. Part A 42, 1764 (2011)
P. Ye, L. Reitz, Ch. Horan, R. Parnas, J. Polym. Environ. 14, 1 (2006)
S. Hemsri, K. Grieco, A.D. Asandei, R.S. Parnas, Compos. Part A 43, 1160 (2012)
B. Winkler, J.K. Margerison, J. Dairy Sci. 95, 1714 (2012)
S. Sharma, I. Luzinov, J. Polym. Environ. 20, 681 (2012)
S. Mallahpour, M. Dinari, J. Inorg. Organomet. Polym. 22, 929 (2012)
R. Kuktaite, H. Ture, M.S. Hedenqvist, M. Gallstedt, T.S. Plivelic, A.C.S. Sustain, Chem. Eng. 2, 1439 (2014)
A. Kiersnowski, M. Serwadczak, E. Kulaga, B. Futoma-Koloch, R. Kwiatkowski, W. Doroszkiewicz, J. Piglowski, Appl. Clay Sci. 44, 225 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wesołowska-Trojanowska, M., Tomczyńska-Mleko, M., Terpiłowski, K. et al. Ternary Biopolymer Based on Wheat Gluten, Whey Protein Concentrate and Montmorillonite. J Inorg Organomet Polym 26, 555–562 (2016). https://doi.org/10.1007/s10904-016-0348-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0348-0