Abstract
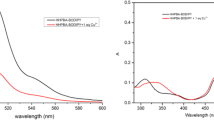
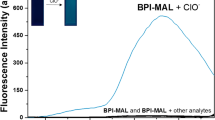
A new boron-dipyrromethene (BODIPY) fluorescent dye aimed at sensitively detecting hypochlorite anion (ClO−) has been designed, synthesized and characterized. The probe is comprised of a BODIPY fluorophore unit and a ClO− specific reactive group of amidoxime. The addition of hypochlorite results in a red-shift of absorption and emission spectra of the probe accompanied by a decrease of intensity and spectra changes (A500 and 1/I512) of the probe can achieve a good linearity to the concentration of ClO−. The fluorescence probe can react to ClO− rapidly (within 60 s) in a wide pH range (4–10) with high sensitivity (detection limit of 6.81 μM) and selectivity. The reaction mechanism has been proposed and confirmed by MS analysis, ClO− anion oxidizes amidoxime moiety to hydroxyl group and hydroxyl group is further oxidized to formyl group in the formation of a corresponding aldehyde compound. In addition, the probe has also been successfully applied to detect ClO− in tap water and river water samples by spiking a known amount of standard ClO−.









Similar content being viewed by others
References
Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ (2006) Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome - implications for microbial killing. J Biol Chem 281(52):39860–39869. https://doi.org/10.1074/jbc.M605898200
Lapenna D, Cuccurullo F (1996) Hypochlorous acid and its pharmacological antagonism: an update picture. General pharmacology: the vascular system 27 (7):1145-1147. https://doi.org/10.1016/S0306-3623(96)00063-8
Prokopowicz ZM, Arce F, Biedron R, Chiang CL-L, Ciszek M, Katz DR, Nowakowska M, Zapotoczny S, Marcinkiewicz J, Chain BM (2010) Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J Immunol 184(2):824–835. https://doi.org/10.4049/jimmunol.0902606
ZgliczyŃSki JM, StelmaszyŃSka T, Ostrowski W, Naskalski J, Sznajd J (1968) Myeloperoxidase of human Leukaemic leucocytes. Eur J Biochem 4(4):540–547. https://doi.org/10.1111/j.1432-1033.1968.tb00246.x
Fiedler TJ, Davey CA, Fenna RE (2000) X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 angstrom resolution. J Biol Chem 275(16):11964–11971. https://doi.org/10.1074/jbc.275.16.11964
Daugherty A, Dunn JL, Rateri DL, Heinecke JW (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94(1):437–444. https://doi.org/10.1172/JCI117342
Wu SM, Pizzo SV (2001) α2-macroglobulin from rheumatoid arthritis synovial fluid: functional analysis defines a role for oxidation in inflammation. Archives of biochemistry and biophysics 391 (1):119-126. https://doi.org/10.1006/abbi.2001.2408
Pullar JM, Vissers MCM, Winterbourn CC (2000) Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life 50(4–5):259–266. https://doi.org/10.1080/15216540051080958
Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3(5):420–425. https://doi.org/10.1093/embo-reports/kvf094
Ramsey MR, Sharpless NE (2006) ROS as a tumour suppressor? Nat Cell Biol 8(11):1213–1215. https://doi.org/10.1038/ncb1106-1215
Aoki T, Munemori M (1983) Continuous-flow determination of free chlorine in water. Anal Chem 55(2):209–212. https://doi.org/10.1021/ac00253a010
Mesquita RBR, Rangel A (2005) Gas diffusion sequential injection system for the spectrophotometric determination of free chlorine with o-dianisidine. Talanta 68(2):268–273. https://doi.org/10.1016/j.talanta.2005.07.028
Odabasi M (2008) Halogenated volatile organic compounds from the use of chlorine-bleach-containing household products. Environmental Science & Technology 42(5):1445–1451. https://doi.org/10.1021/es702355u
Chen X, Tian X, Shin I, Yoon J (2011) Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev 40(9):4783–4804. https://doi.org/10.1039/c1cs15037e
Fernandez-Suarez M, Ting AY (2008) Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol 9(12):929–943. https://doi.org/10.1038/nrm2531
Yue Y, Dong Q, Zhang Y, Sun Y, Gong Y (2015) A highly selective "turn-on" fluorescent chemosensor based on 8-aminoquinoline for detection of Zn2+. Anal Methods 7(13):5661–5666. https://doi.org/10.1039/c5ay01007a
Hudnall TW, Gabbai FP (2008) A BODIPY boronium cation for the sensing of fluoride ions. Chem Commun 38:4596–4597. https://doi.org/10.1039/b808740g
Cao X, Lin W, Zheng K, He L (2012) A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem Commun 48(85):10529–10531. https://doi.org/10.1039/c2cc34031c
Liu J, Yue Y, Wang J, Yan X, Liu R, Sun Y, Li X (2015) Study of interaction between human serum albumin and three phenanthridine derivatives: fluorescence spectroscopy and computational approach. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy 145:473–481. https://doi.org/10.1016/j.saa.2015.03.069
Vernekar SKV, Hallaq HY, Clarkson G, Thompson AJ, Silvestri L, Lummis SCR, Lochner M (2010) Toward biophysical probes for the 5-HT3 receptor: structure-activity relationship study of Granisetron derivatives. J Med Chem 53(5):2324–2328. https://doi.org/10.1021/jm901827x
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41(3):1130–1172. https://doi.org/10.1039/c1cs15132k
Kenmoku S, Urano Y, Kojima H, Nagano T (2007) Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J Am Chem Soc 129(23):7313–7318. https://doi.org/10.1021/ja068740g
Liu S-R, Wu S-P (2013) Hypochlorous acid turn-on fluorescent probe based on oxidation of diphenyl selenide. Org Lett 15(4):878–881. https://doi.org/10.1021/ol400011u
Lou Z, Li P, Song P, Han K (2013) Ratiometric fluorescence imaging of cellular hypochlorous acid based on heptamethine cyanine dyes. Analyst 138(21):6291–6295. https://doi.org/10.1039/c3an00198a
Zha J, Fu B, Qin C, Zeng L, Hu X (2014) A ratiometric fluorescent probe for rapid and sensitive visualization of hypochlorite in living cells. RSC Adv 4(81):43110–43113. https://doi.org/10.1039/c4ra07009g
Chen X, Wang X, Wang S, Shi W, Wang K, Ma H (2008) A highly selective and sensitive fluorescence probe for the hypochlorite anion. Chem Eur J 14(15):4719–4724. https://doi.org/10.1002/chem.200701677
Yin W, Zhu H, Wang R (2014) A sensitive and selective fluorescence probe based fluorescein for detection of hypochlorous acid and its application for biological imaging. Dyes Pigments 107:127–132. https://doi.org/10.1016/j.dyepig.2014.03.012
Cheng X, Jia H, Long T, Feng J, Qin J, Li Z (2011) A "turn-on" fluorescent probe for hypochlorous acid: convenient synthesis, good sensing performance, and a new design strategy by the removal of C = N isomerization. Chem Commun 47(43):11978–11980. https://doi.org/10.1039/c1cc15214a
Chen W-C, Venkatesan P, Wu S-P (2015) A highly selective turn-on fluorescent probe for hypochlorous acid based on hypochlorous acid-induced oxidative intramolecular cyclization of boron dipyrromethene-hydrazone. Anal Chim Acta 882:68–75. https://doi.org/10.1016/j.aca.2015.04.012
Lin W, Long L, Chen B, Tan W (2009) A Ratiometric fluorescent probe for hypochlorite based on a Deoximation reaction. Chem Eur J 15(10):2305–2309. https://doi.org/10.1002/chem.200802054
Cheng G, Fan J, Sun W, Sui K, Jin X, Wang J, Peng X (2013) A highly specific BODIPY-based probe localized in mitochondria for HClO imaging. Analyst 138(20):6091–6096. https://doi.org/10.1039/C3AN01152F
Emrullahoglu M, Ucuncu M, Karakus E (2013) A BODIPY aldoxime-based chemodosimeter for highly selective and rapid detection of hypochlorous acid. Chem Commun 49(71):7836–7838. https://doi.org/10.1039/C3CC44463E
Yu S-Y, Hsu C-Y, Chen W-C, Wei L-F, Wu S-P (2014) A hypochlorous acid turn-on fluorescent probe based on HOCl-promoted oxime oxidation and its application in cell imaging. Sensors and Actuators B-Chemical 196:203–207. https://doi.org/10.1016/j.snb.2014.01.121
Aubry JM, Cazin B, Duprat F (1989) Chemical sources of singlet oxygen .3. Peroxidation of water-soluble singlet oxygen carriers with the hydrogen-peroxide molybdate system. J Org Chem 54(3):726–728. https://doi.org/10.1021/jo00264a046
Umezawa N, Tanaka K, Urano Y, Kikuchi K, Higuchi T, Nagano T (1999) Novel fluorescent probes for singlet oxygen. Angewandte Chemie-International Edition 38(19):2899–2901. https://doi.org/10.1002/(sici)1521-3773(19991004)38:19<2899::aid-anie2899>3.0.co;2-m
Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T (2003) Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem 278(5):3170–3175. https://doi.org/10.1074/jbc.M209264200
Fenton HJH (1894) LXXIII.-oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65 (0):899–910. https://doi.org/10.1039/CT8946500899
Sun Z-N, Liu F-Q, Chen Y, Tam PKH, Yang D (2008) A highly specific BODIPY-based fluorescent probe for the detection of hypochlorous acid. Org Lett 10(11):2171–2174. https://doi.org/10.1021/ol800507m
Chen Y, Jiang J (2011) 4-(4,4-Difluoro-1,3,5,7-tetramethyl-3a-aza-4a-azonia-4-borata-s-indacen-8-yl)benzonitrile. Acta Crystallographica Section E-Structure Reports Online 67:O908–U831. https://doi.org/10.1107/s1600536811009457
Srivastava RM, Brinn IM, MachucaHerrera JO, Faria HB, Carpenter GB, Andrade D, Venkatesh CG, deMorais LPF (1997) Benzamidoximes: Structural, conformational and spectroscopic studies .1. J Mol Struct 406(1–2):159–167. https://doi.org/10.1016/s0022-2860(96)09452-5
S-s K, H-s Z, H-l L, Wang H-b, Wang P-l (2007) 4-chlorobenzamidoxime. Acta Crystallographica Section E-Structure Reports Online 63:O4698–U4937. https://doi.org/10.1107/s1600536807057273
Long GL, Winefordner JD (1983) Limit of detection. Analytical chemistry 55 (7):A712−+. https://doi.org/10.1021/ac00258a001
Goswami S, Maity S, Maity AC, Das AK (2014) Fluorometric and naked-eye detectable dual signaling chemodosimeter for hypochlorite. Sensors Actuators B Chem 204:741–745. https://doi.org/10.1016/j.snb.2014.08.024
Goswami S, Paul S, Manna A (2013) Highly reactive (<1 min) ratiometric "naked eye" detection of hypochlorite with real application in tap water. Dalton Trans 42(28):10097–10101. https://doi.org/10.1039/C3DT51238J
Li G, Ji D, Zhang S, Li J, Li C, Qiao R (2017) A mitochondria-targeting fluorescence turn-on probe for hypochlorite and its applications for in vivo imaging. Sensors Actuators B Chem 252:127–133. https://doi.org/10.1016/j.snb.2017.05.138
Li J, Yin C, Liu T, Wen Y, Huo F (2017) A new mechanism-based fluorescent probe for the detection of ClO− by UV–vis and fluorescent spectra and its applications. Sensors and actuators B: chemical 252 (supplement C):1112-1117. https://doi.org/10.1016/j.snb.2017.07.171
Reja SI, Bhalla V, Sharma A, Kaur G, Kumar M (2014) A highly selective fluorescent probe for hypochlorite and its endogenous imaging in living cells. Chem Commun 50(80):11911–11914. https://doi.org/10.1039/C4CC05356G
Shen S-L, Zhang X-F, Ge Y-Q, Zhu Y, Cao X-Q (2018) A novel ratiometric fluorescent probe for the detection of HOCl based on FRET strategy. Sensors and actuators B: chemical 254 (supplement C):736-741. https://doi.org/10.1016/j.snb.2017.07.158
Tang Z, Ding X-L, Liu Y, Zhao Z-M, Zhao B-X (2015) A new probe based on rhodamine B and benzothiazole hydrazine for sensing hypochlorite in living cells and real water samples. RSC Adv 5(121):99664–99668. https://doi.org/10.1039/C5RA20188H
Wang Q, Liu C, Chang J, Lu Y, He S, Zhao L, Zeng X (2013) Novel water soluble styrylquinolinium boronic acid as a ratiometric reagent for the rapid detection of hypochlorite ion. Dyes and pigments 99(3):733–739. https://doi.org/10.1016/j.dyepig.2013.06.019
Yuan L, Lin W, Song J, Yang Y (2011) Development of an ICT-based ratiometric fluorescent hypochlorite probe suitable for living cell imaging. Chem Commun 47(47):12691–12693. https://doi.org/10.1039/C1CC15762K
Gai L, Mack J, Liu H, Xu Z, Lu H, Li Z (2013) A BODIPY fluorescent probe with selective response for hypochlorous acid and its application in cell imaging. Sensors and actuators B: chemical 182:1–6. https://doi.org/10.1016/j.snb.2013.02.106
Shi J, Li Q, Zhang X, Peng M, Qin J, Li Z (2010) Simple triphenylamine-based luminophore as a hypochlorite chemosensor. Sensors and actuators B: chemical 145(1):583–587. https://doi.org/10.1016/j.snb.2009.11.003
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21571088)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Table S1
Comparison of probes for the detection of ClO- (DOCX 12 kb)
Fig. S1
Plot of fluorescence intensity I (at 512 nm) versus [ClO−] for probe AO-BODIPY (5 μM) in HEPES-EtOH solution (20 mM HEPES, 1/9, v/v, pH = 7.0, λex = 470 nm) (PNG 85 kb)
Fig. S2
The normalized absorption and emission spectra of AO-BODIPY (5 μM) in HEPES-EtOH solution (20 mM HEPES, 1/9, v/v, pH = 7.0, λex = 470 nm) (PNG 107 kb)
Fig. S3
The absorbance of AO-BODIPY (5 μM) at 500 nm as a function of concentrations of ClO- in HEPES-EtOH solution (20 mM HEPES, 1/9, v/v, pH = 7.0) (PNG 98 kb)
Fig. S4
Absorption spectra of AO-BODIPY (5 μM) upon addition of different ions and ROS (20 equiv) in HEPES-EtOH solution (20 mM HEPES, 1/9, v/v, pH = 7.0) (PNG 212 kb)
Fig. S5
The ESI-MS spectrum of the products separated from the reaction of AO-BODIPY with NaClO (10 equiv) in HEPES-EtOH solution (20 mM HEPES, 1/9, v/v, pH = 7.0) at room temperature (PNG 1396 kb)
Fig. S6
The 1H NMR spectrum of AO-BODIPY in d6-DMSO (PNG 411 kb)
Fig. S7
The 13C NMR spectrum of AO-BODIPY in d6-DMSO (PNG 397 kb)
Fig. S8
The HRMS spectrum of AO-BODIPY (PNG 435 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Li, B., Jiang, C. et al. A BODIPY Based Fluorescent Probe for the Rapid Detection of Hypochlorite. J Fluoresc 28, 933–941 (2018). https://doi.org/10.1007/s10895-018-2255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2255-y