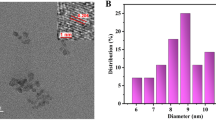
Abstract
Carbon dots has becoming one of the most promising fluorescence sensors to determine the trace level of heavy metals in environments because of their advantages in optical properties, response time, and convenient operation procedures. Herein, a novel nitrogen and sulfur co-doped carbon dots (NS-CDs) were prepared though microwave assisted approach using DL-malic acid and allyl thiourea for the first time. Due to the existence of nitrogen and sulfur, the as-prepared NS-CDs exhibited bright blue fluorescence at 430 nm upon 330 nm excitation, with a fluorescence quantum yield of 19.8%. The sensitivity study of NS-CDs against metal ions and organic molecules has approved that the fluorescence could be further quenched by Ce4+ and Fe3+ ions, with the same linear detection ranges varying from 10 to 90 µM. The limits of detection (LOD) were determined as low as 0.75 µM and 0.67 µM for Ce4+ and Fe3+ ions, respectively. The possible quenching mechanism is explained by inner filter effect and static quenching mechanism for Ce4+ ions, while the quenching effect caused by Fe3+ ions is attributed to the inner filter effect, static and dynamic quenching mechanisms. Additionally, the developed sensor was used for the detection of Ce4+ and Fe3+ ions in tap water with satisfactory recoveries. Finally, the designed NS-CDs sensor possesses good biocompatibility against MA104 cells, suggesting the sensor can be potentially applied to detect Ce4+ and Fe3+ ions in environment and biological systems.










Similar content being viewed by others
Data Availability
The datasets supporting the results during the current study are available from the corresponding author on reasonable request.
References
Bordoloi P et al (2022) Condensation Product of 1-Naphthaldehyde and 3-Aminophenol: Fluorescent “on” Probe for Ce3+and “off” Probe for Dichromate (Cr2O72−). J Fluoresc 32:1189–1198. https://doi.org/10.1007/s10895-022-02927-0
Han SJ et al (2022) Fluorometric and colorimetric detection of cerium(IV) ion using carbon dots and bathophenanthroline-disulfonate-ferrum(II) complex. Spectrochim Acta Part A-Mol Biomol Spectrosc 264:122095. https://doi.org/10.1016/j.saa.2021.120295
Li X et al (2022) Nitrogen and boron co-doped carbon dots as a novel fluorescent probe for fluorogenic sensing of Ce4+ and ratiometric detection of Al3+. Spectrochim Acta Part A Mol Biomol Spectrosc 282:121638. https://doi.org/10.1016/j.saa.2022.121638
Xia M et al (2020) Graphene quantum dots combined with the oxidase-mimicking activity of Ce4+ for ratiometric fluorescent detection of Ce4+ and alendronate sodium. Sens Actuators, B Chem 319:128321. https://doi.org/10.1016/j.snb.2020.128321
Nemati R, Zare-Dorabei R (2019) A ratiometric probe based on Ag2S quantum dots and graphitic carbon nitride nanosheets for the fluorescent detection of Cerium. Talanta 200:249–255. https://doi.org/10.1016/j.talanta.2019.03.059
Chen J et al (2018) Stripping voltammetric determination of cerium in food using an electropolymerized poly-catechol and ion-imprinted membrane modified electrode. J Electroanal Chem 808:41–49. https://doi.org/10.1016/j.jelechem.2017.11.049
Ghosh M et al (2018) Recognition of ceric ion in aqueous medium at pico-molar level: Colorimetric, fluorimetric and single crystal X-ray structural evidences. J Photochem Photobiol, A 367:32–38. https://doi.org/10.1016/j.jphotochem.2018.08.010
Cui J et al (2022) N-doped carbon dots as fluorescent “turn-off” nanosensors for ascorbic acid and Fe3+ detection. ACS Appl Nano Mater 5:7268–7277. https://doi.org/10.1021/acsanm.2c01170
Singh S, Kansal SK (2022) Dual fluorometric detection of Fe3+ and Hg2+ ions in an aqueous medium using carbon quantum dots as a “turn-off” fluorescence sensor. J Fluoresc 32:1143–1154. https://doi.org/10.1007/s10895-022-02922-5
Liu H, Haoxuan Xu, Li H (2022) detection of Fe3+ and Hg2+ ions by using high fluorescent carbon dots doped with S and N as fluorescence probes. J Fluoresc 32:1089–1098. https://doi.org/10.1007/s10895-022-02921-6
Nagaraj M et al (2022) Detection of Fe3+ ions in aqueous environment using fluorescent carbon quantum dots synthesized from endosperm of Borassus flabellifer. Environ Res 212:113273. https://doi.org/10.1016/j.envres.2022.113273
Eisenstein RS, Blemings KP (1998) Iron responsive elements and iron homeostasis. J Nutr Blemings Iron Regul Proteins 128:2295
Torti SV, Torti FM (2013) Iron and cancer: more ore to be mined. Nat Rev Cancer 13:342–355. https://doi.org/10.1038/nrc3495
Tabaraki R, Rahmatinya Z (2021) Bifunctional nitrogen and fluorine co-doped carbon dots as fluorescence probe for silicon and mercury by pH switching. J Fluoresc 31:881–887. https://doi.org/10.1007/s10895-021-02709-0
Luo Q et al (2021) Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chem Eng J 407:127050. https://doi.org/10.1016/j.cej.2020.127050
Wang R et al (2021) Simple and green synthesis of carbonized polymer dots from nylon 66 waste fibers and its potential application. ACS Omega 48:32888–32895. https://doi.org/10.1021/acsomega.1c04808
Wu Y et al (2022) Preparation of carbon dots with ultrahigh fluorescence quantum yield based on PET waste. ACS Omega 42:38037–38044. https://doi.org/10.1021/acsomega.2c05324
Mejía ÁJ et al (2022) Avocado seeds derived carbon dots for highly sensitive Cu (II)/Cr (VI) detection and copper (II) removal via flocculation. Chem Eng J 446:137171. https://doi.org/10.1016/j.cej.2022.137171
Gu W et al (2022) Functionalization of PET with carbon dots as copolymerizable flame retardants for the excellent smoke suppressants and mechanical properties. Polym Degrad Stab 195:109766. https://doi.org/10.1016/j.polymdegradstab.2021.109766
Gu W et al (2022) Carbon Dots as smoke suppression agents for the reduction of CO release in combustion and improvement of UV resistance towards Phosphorus-containing polyester. Eur Polymer J 181:111642. https://doi.org/10.1016/j.eurpolymj.2022.111642
Zhang Y et al (2021) Yttrium-mediated red fluorescent carbon dots for sensitive and selective detection of calcium ions. 36:1969–1976. https://doi.org/10.1002/bio.4132
Zhang Z et al (2020) A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale 12:13899–13906. https://doi.org/10.1039/D0NR03163A
Abbasi A, Shakir M (2022) Simple one-step solid-state synthesis of highly crystalline N doped carbon dots as selective turn off-sensor for picric acid and metanil yellow. J Fluoresc 32:1239–1246. https://doi.org/10.1007/s10895-022-02928-z
Shihai M et al (2020) Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 33:100879. https://doi.org/10.1016/j.nantod.2020.100879
Yan Z et al (2022) One-step synthesis of nitrogen/fluorine co-doped carbon dots for use in ferric ions and ascorbic acid detection. Nanomaterials 12:2377. https://doi.org/10.3390/nano12142377
Galal M et al (2021) Green one-pot synthesis of nitrogen and sulfur co-doped carbon quantum dots as new fluorescent nanosensors for determination of salinomycin and maduramicin in food samples. Food Chem 343:128539. https://doi.org/10.1016/j.foodchem.2020.128539
Kou X et al (2020) A review: recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans 49:6915–6938. https://doi.org/10.1039/D0DT01004A
Largitte L et al (2021) Effect of the surface chemistry on the photoluminescence properties of boron doped carbon dots. J Photochem Photobiol, A 405:112903. https://doi.org/10.1016/j.jphotochem.2020.112903
Shen Y et al (2022) Graphene oxide-assisted synthesis of N, S Co-doped carbon quantum dots for fluorescence detection of multiple heavy metal ions. Talanta 241:123224. https://doi.org/10.1016/j.talanta.2022.123224
El-Malla SF et al (2022) Rapid microwave synthesis of N, S-doped carbon quantum dots as a novel turn off-on sensor for label-free determination of copper and etidronate disodium. Anal Chim Acta 1197:339491. https://doi.org/10.1016/j.aca.2022.339491
Mintz KJ et al (2021) A deep investigation into the structure of carbon dots. Carbon 173:433–447. https://doi.org/10.1016/j.carbon.2020.11.017
Bai L et al (2019) Multi-excitation and single color emission carbon dots doped with silicon and nitrogen: Synthesis, emission mechanism, Fe3+ probe and cell imaging. Chem Eng J 373:963–972. https://doi.org/10.1016/j.cej.2019.05.103
Song Z et al (2016) Multifunctional N, S co-doped carbon quantum dots with pH- and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 104:169–178. https://doi.org/10.1016/j.carbon.2016.04.003
Lu W et al (2015) Comparative study for N and S doped carbon dots: synthesis, characterization and applications for Fe3+ probe and cellular imaging. Anal Chim Acta 898:116–127. https://doi.org/10.1016/j.aca.2015.09.050
Keerthana P et al (2022) Detection of picric acid in industrial effluents using multifunctional green fluorescent B/N-carbon quantum dots. J Environ Chem Eng 10:107209. https://doi.org/10.1016/j.jece.2022.107209
Mahmoud AM et al (2022) Fluorometric and electrochemical dual-mode detection of toxic flavonoid rutin based on new nitrogen and sulfur co-doped carbon dots: Enhanced selectivity based on masking the interfering flavonoids with BSA complexation. J Food Compos Anal 108:104428. https://doi.org/10.1016/j.jfca.2022.104428
Wu X et al (2022) F, N-Doped carbon dots as efficient Type I photosensitizers for photodynamic therapy. Dalton Trans 51:2296–2303. https://doi.org/10.1039/D1DT03788A
Dang DK et al (2018) One pot solid-state synthesis of highly fluorescent N and S co-doped carbon dots and its use as fluorescent probe for Ag+ detection in aqueous solution. Sens Actuators, B Chem 255:3284–3291. https://doi.org/10.1016/j.snb.2017.09.155
Liu W et al (2017) Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens Actuators, B Chem 241:190–198. https://doi.org/10.1016/j.snb.2016.10.068
Cheng R et al (2021) Dimensional engineering of carbon dots derived sulfur and nitrogen co-doped carbon as efficient oxygen reduction reaction electrocatalysts for aluminum-air batteries. Chem Eng J 425:130603. https://doi.org/10.1016/j.cej.2021.130603
Liu G et al (2018) Facile synthesis of nitrogen and sulfur co-doped carbon dots for multiple sensing capacities: alkaline fluorescence enhancement effect, temperature sensing, and selective detection of Fe3+ ions. New J Chem 42:13147–13156. https://doi.org/10.1039/C8NJ02086H
Huang J et al (2021) Nitrogen and chlorine co-doped carbon dots with synchronous excitation of multiple luminescence centers for blue-white emission. New J Chem 45:7056–7059. https://doi.org/10.1039/D1NJ00951F
Sharma A et al (2016) Origin of excitation dependent fluorescence in carbon nanodots. The Journal of Physical Chemistry Letters 7:3695–3702. https://doi.org/10.1021/acs.jpclett.6b01791
Van Dam B et al (2017) Excitation-dependent photoluminescence from single-carbon dots. Small 13:1702098. https://doi.org/10.1002/smll.201702098
Yang J et al (2021) Facile and green synthesis of bifunctional carbon dots for detection of Cu2+ and ClO– in aqueous solution. ACS Sustain Chem Eng 9:13206–13214. https://doi.org/10.1021/acssuschemeng.1c03868
Chun L et al (2018) Excitation dependent emission combined with different quenching manners supports carbon dots to achieve multi-mode sensing. Sens Actuators, B Chem 263:1–9. https://doi.org/10.1016/j.snb.2018.02.050
Zhu J et al (2021) Nitrogen and fluorine co-doped green fluorescence carbon dots as a label-free probe for determination of cytochrome c in serum and temperature sensing. J Colloid Interface Sci 586:683–691. https://doi.org/10.1016/j.jcis.2020.10.138
Liu Y et al (2021) Nitrogen and sulfur co-doped carbon dots: Facile synthesis and multifunctional applications for pH sensing, temperature sensing and RNA-selective imaging. Microchem J 168:106248. https://doi.org/10.1016/j.microc.2021.106248
Chu Xu, Wang S, Cao Y (2020) A new fluorescence probe comprising nitrogen-doped graphene quantum dots for the selective and quantitative determination of cerium(iv). New J Chem 44:797–806. https://doi.org/10.1039/C9NJ04518J
Yan F et al (2019) Synthesis and spectral analysis of fluorescent probes for Ce4+ and OCl− ions based on fluorescein Schiff base with amino or hydrazine structure: Application in actual water samples and biological imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 213:254–262. https://doi.org/10.1016/j.saa.2019.01.045
Long C et al (2021) Applications of carbon dots in environmental pollution control: A review. Chem Eng J 406:126848. https://doi.org/10.1016/j.cej.2020.126848
Jiang W et al (2021) Carbon dot-based biosensors. Adv NanoBiomed Res 1:2000042. https://doi.org/10.1002/anbr.202000042
Li X et al (2021) Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord Chem Rev 431:213686. https://doi.org/10.1016/j.ccr.2020.213686
Zeng Y et al (2022) Novel N, F co-doped carbon dots to detect sulfide and cadmium ions with high selectivity and sensitivity based on a “turn-off-on” mechanism. Dyes Pigm 203:110379. https://doi.org/10.1016/j.dyepig.2022.110379
Zhao Q et al (2022) Surface amino group modulation of carbon dots with blue, green and red emission as Cu2+ ion reversible detector. Appl Surf Sci 598:153892. https://doi.org/10.1016/j.apsusc.2022.153892
Bandi R et al (2018) Green synthesis of highly fluorescent nitrogen – Doped carbon dots from Lantana camara berries for effective detection of lead(II) and bioimaging. J Photochem Photobiol, B 178:330–338. https://doi.org/10.1016/j.jphotobiol.2017.11.010
Shangguan J et al (2017) Highly Fe3+ selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal Chem 89:7477–7484. https://doi.org/10.1021/acs.analchem.7b01053
Zhang Li et al (2021) 11-Mercaptoundecanoic acid-functionalized carbon dots as a ratiometric optical probe for doxorubicin detection. ACS Applied Nano Materials 4:13734–13746. https://doi.org/10.1021/acsanm.1c03141
Funding
This work was supported by National Natural Science of China (Grant numbers 52071281 and 51971197), the Natural Science Foundation of Hebei Province (Grant numbers E2020203081 and B2022407009), the Science and Technology Project of Hebei Education Department (Grant number BJK2022033), and the Hebei Province Foundation for Returned Talent (Grant number C20210322).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Lichao Pei: Investigation, Methodology, Writing – review & editing. Weiyuan Zhang: Investigation, Methodology, review & editing. Shuqin Yang: Investigation, review & editing. Kangli Chen: Investigation, Methodology, review & editing. Xiaoxun Zhu: Investigation, Methodology. Yan Zhao: Review & editing. Shumin Han: Review & editing. All authors have read and approved the final manuscript, experiments and original draft.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, L., Zhang, W., Yang, S. et al. Nitrogen and Sulfur Co-doped Carbon Dots as a Turn-Off Fluorescence Probe for the Detection of Cerium and Iron. J Fluoresc 33, 1147–1156 (2023). https://doi.org/10.1007/s10895-022-03126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03126-7