Abstract
An electroantennogram (EAG) technique compared the antennal olfactory responses by both sexes of eight Japanese Papilio species with known host plants in laboratory experiments. Papilio species were collected from Honshû and Kyûshû (Japanese islands). The behavioral responses to volatile leaf substances from Citrus deliciosa, Zanthoxylum ailanthoides, Phellodendron amurense, Orixa japonica, and Foeniculum vulgare were examined in laboratory experiments. Individual EAG reactions were recorded. The results were very similar to the empirical field observations. The electrophysiological results of both sexes showed that the volatile substances released from non-preferred plants mainly elicited more significant EAG responses than the volatile substances from preferred host plants. Moreover, we performed behavioral experiments using eight female butterflies and their responses to five host plant species. An association between host plant selection behavior and taxonomical classification exists within the Papilio genus. The EAG responses were small when exposed to the plants with high scores in the behavioral experiments. Host plant preference patterns seem to be related to the volatile substances within the host plants. The butterflies responded to Linalool in both the behavioral and electrophysiological experiments.
Similar content being viewed by others
Introduction
Papilio female butterflies verify hostplants using their foretarsal contact chemosensillar (Niki and Kanzaki 1995; Honda et al. 2011). Female butterflies also find hostplants by utilizing the various odors rising from the hostplant leaf surface. Inoue et al. (2023) identified the significant odorants of 14 hostplants of Japanese Papilio species. Moreover, Inoue et al. (2019) examined the morphology of the antennae of Papilio and demonstrated that many olfactory sensilla exist with hygroreceptive sensilla.
A literature search (e.g., Fukuda et al. 1982; Nihira 2004; Inoue 2013; shown in Table 1) revealed eight Papilio species on the Japanese mainland (Honshû and Kyûshû) exhibit the same patterns in hostplant selection. Japanese Papilio species in Honshû and Kyûshû are split into two groups by their preference for Citrus ssp. One group comprises P. xuthus, P. helenus, P. protenor, and P. memnon, with females preferring Citrus ssp. to other Rutaceae and Apiaceae plants. The other group, which is comprised of P. maackii, P. bianor, P. macilentus, and P. machaon prefer other host plants, such as Z. ailanthoides, P. amurense, O. japonica, or Apiaceae plants. We hypothesized that the preferences of Japanese Papilio species are attributed to plant volatile substances. To investigate this hypothesis, we examined the behavioral and electrophysiological responses of the eight Japanese Papilio species to the volatile substances of their host plants and other related plants.
A study by Inoue et al. (2023) described, Linalool can be detected in the flowers of all six plant species examined; therefore, behavioral experiments on Linalool and electrophysiological experiments by EAG or Single sensillum recording were conducted.
Materials and Methods
Butterflies
The butterflies used in the experiments were reared in our laboratory in Tsukuba City, Ibaraki, or collected from the field. Field collections of butterflies not protected by Japanese and international laws were sampled from locations that do not require any specific entrance or collection permits. Collected butterflies were used in the experiments, and female butterflies were also used to obtain their eggs. Hatched larvae and larvae collected from the field were reared in our laboratory in Tsukuba City. The larvae of P. xuthus, P. helenus, P. protenor were fed Z. ailanthoides. P. amurense was fed to P. maackii larvae. O. japonica was fed to P. bianor and P. macilentus larvae. Citrus spp. was fed to P. memnon larvae. F. vulgare was fed to P. machaon larvae. These feed plants are the respective hostplants for each of the butterfly species. All experiments were performed in the same laboratory. Immediately after the butterfly emergence or arrival in the experimental room, the female butterflies were stored in rearing cages (500 mm width × 500 mm depth × 700 mm height) that were covered with semitransparent nylon organdy. A 20 mL polyethylene tube filled with Pokari Sweat (Otsuka Pharmaceutical, Tokyo, Japan; an isotonic sports drink) was placed in each rearing cage, and an artificial flower petal made of nylon cloth was tethered from the cage ceiling (Fig. 1). The Pokari Sweat was soaked through the tube onto the upper side of the petal along a paper string that pierced the artificial petal. This feeding design enabled the butterflies to consume the Pokari Sweat as required. Butterflies grown in our laboratory were used within seven days of the emergency, and those captured from the fields were used within two days of their initial capture. After the experiments, most butterflies were released, and the remaining few were reared to obtain the next generation. We performed behavioral experiments using eight female butterflies and their eight hostplant species. Butterflies collected in the field were returned to their natural habitat, and those reared in rearing cages were returned there.
Behavioral Experiments
Butterflies and the Experimental Cage
We initially traditionally attempted the behavioral experiment using a T-maze or Y-maze, but all these trials were unsuccessful. In our previous laboratory experiments (not published), the female Papilio butterflies seemed to require flying space to perform their normal oviposition behavior. Therefore, we created a well-suited wind cage for our purpose (Figs. 2 and 3). The cage was rectangular parallel pipes 500 mm wide × 500 mm deep × 700 mm high. This cage had a steel frame and a transparent chlorinated vinyl sheet cover. Two fans, 120 mm in diameter (Genuine Globe Fan, RL4R S1202512L-3 M 120 mm Case Fan, DC 12 V, 0.26 A, Sleeve Bearing) were mounted in two holes (100 mm in diameter) on the ceiling, and two holes (80 mm in diameter) for air intake were made right below each fan on the floor. The holes were covered with acryl plates perforated with many holes (5 mm in diameter). The cage floor was placed 200 mm above the cage bottom to provide space for airflow (Fig. 2a). Cups were placed under each hole in the floor (Fig. 2b). One cup was empty (a control), and the other was filled with 10 g of chopped plant leaves. The volatile substances released from the cut leaves are dispersed into the cage by the air current produced by the fan. The outside air was also dispersed using the same fan technique (Fig. 3). A single butterfly was transferred from the rearing cage into the experimental cage, and its behavior was observed. Our previous behavioral experiments showed that all Papilio butterflies seemed to possess good memories Therefore, all butterflies were only used once in any behavioral experiment (Fig. 4).
Experimental design of the behavioral experiments cage. a: entire panoramic view of the cage. b: the cage in progress, the wind intake and exhaust are compacting the sides. The water and dry-ice mixture were in the two cups set into the floor of the cage and two water smoke pillars (indicated by yellow arrows) were straightened by the fans attached to the ceiling of the cage
Cage in progress. Two artificial branches containing leaves were placed in the wind flows created by the two fans. Female butterflies were transferred into the cage. a: this female was given a behavioral score 0, as it landed immediately after being introduced into the cage. b: this female was given a behavioral score of 2, as it laid eggs
Scoring of Female Butterfly Behavior
First, we observed the behavior of each butterfly in response to the female-preferred host plant. Specifically, we examined the behavior of P. xuthus, P. helenus, P. protenor, and P. memnon to plants of C. deliciosa, P. maackii to P. amurense, P. bianor and P. macilentus to O. japonica, and P. machaon to F. vulgare. The female butterflies that did not exhibit any response by landing on the cage floor were not used in the experiment. Only butterflies that showed flight behavior was used in the experiment. We performed the same experiments using four other plant species and rechecked the behavior toward the initial hostplant. If the final test score was consistent with the initial response, the data was retained and the individual was not used again.
The placement of the artificial plant leaves in the air flow enabled the plant’s volatile substances to diffuse throughout the cage. The female butterflies began searching for behavior immediately after they entered the cage with the volatile substance. We scored butterfly behavior as follows: Score 0, butterfly exhibited no searching behavior and landed on the cage floor; Score 1, butterfly took off from the floor and began a search flight; Score 2, butterfly took off and after a search flight, showed oviposition behavior on the artificial plant in an air current containing the plant volatile. When multiple behaviors were difficult to distinguish, we scored 0.5 for Scores 0 and 1 and 1.5 for Scores 1 and 2. The behavioral experiments were performed from 2016 to 2018.
Electrophysiology
Stimulation
The set-up of the electrophysiological equipment is provided in Fig. 5a. The control and stimulant carrying air were prepared as follows. Air was pumped and cleaned through bottles containing active carbon and dried through bottles containing silica gel. The air passed into two tubes. Air in one tube was used as the control, and air in the other tube was used as the stimulant carrier. Each airline was sent to a three-way electromagnetic air valve regulated by a programmable electric stimulator (SEN-7203 Nihon Kohden, Tokyo, Japan) or PowerLab (Pl3504, ADInstruments, Colorado Spring, CO, USA). One outlet of the valve was used as the control airline by connecting it to a glass tube (Fig. 5b-2, diameter 7 mm) with the apex positioned 15 mm from one of the butterfly’s antennae. The other outlet was connected to a serially concatenated manual 3-way valve. In some of the experiments, each outlet was connected to a Pasteur pipette via a silicon tube (Fig. 5b-3), while in the other experiments, each outlet was connected to a specific Pasteur pipette via a gas wash bottle. In the former method, a small filter paper was soaked in a stimulant solution and inserted into each Pasteur pipette. The tip of the pipette was positioned 15 mm from an antenna of the butterfly. In the second method, each bottle contained 1 g of leaf chips (approximately 5 mm square pieces) as the source of the plant’s volatile substances, and its outlet was connected to a glass tube (7 mm in diameter). The apex of the tube was positioned 15 mm from the butterfly antenna. The stimulants were manually changed using the 3-way valve.
Electrophysiological equipment built in the laboratory of the Department of Earth System Science, Fukuoka University. a: panoramic view of the electrical shield cage, b: connection tubing, c: entire view of the butterfly preparation stage, d: preparation platform in use. 1: 3-way electromagnetic air valve, 2: control air glass pipe, 3: air separator (serially concatenated manual 3-way valves), 4: glass bottles containing chopped leaf pieces, 5: pipe end, positioned 15 mm from the butterfly’s antenna, 6: preparation platform, 7: rectangular hole for the butterfly’s body, 8: short platinum wire used as the recording electrode, 9: the indifferent electrode
Response Recording
A preparation platform was constructed from an acrylic plate (Fig. 5c—6, 80 mm × 80 mm × 5 mm) with a rectangular hole (Fig. 5c-7, 25 mm × 7 mm) in the center for the butterfly body. A thin layer of paraffin with a low melting point was laid in the center of the platform. The body of an anesthetized butterfly using ice was placed in the hole. The butterfly wings were outstretched on the platform before the butterfly was covered with an acrylic plate to prevent wing and body movement. Two thin grooves were sculpted along the antennae with an inoculation needle and lowered on a thin paraffin layer on the platform. The antennae were fixed in the punctiform along the grooves with the same paraffin on the platform. A short platinum wire was placed on the platform (Fig. 5c – 8), and an antenna was placed using a small volume of conductive cream on the platinum wire, which was used as an active electrode. As an indifferent electrode, a small platinum plate was placed near the butterfly’s head with a small piece of cotton soaked in a physiological salt solution (Fig. 5c - 9).
The active electrode was connected to a high-impedance amplifier (JZ-802; Nihon Kohden, Tokyo, Japan), and the output signal was amplified with a DC-AC amplifier (EX1; Dagan Corporation, Minneapolis, MN, USA). The amplified signal was sent to a data acquisition system (PowerLab, ADInstruments) and an oscilloscope for monitoring. We recorded and saved the electroantennogram (EAG) responses from the butterfly’s antennae. For each plant leaf volatile, an antenna was stimulated three times for 3 s durations with 7 s intervals and then left to rest without any stimulation for 3 min before beginning the stimulation with a new plant volatile. Each butterfly was tested with the five major hostplants for at least one Papilio species on the Japanese mainland. Plants used in these experiments were C. deliciosa (Rutaceae), Z. ailanthoides (Rutaceae), P. amurense (Rutaceae), O. japonica (Rutaceae), and F. vulgare (Apiaceae).
Although male butterflies do not lay eggs, they were often observed flying around the host plants (Yokohari et al. 2017) and displaying a strong interest. Therefore, we also examined male butterflies' electroantennogram (EAG) responses to plant volatile substances. The response magnitude was evaluated as the difference between the voltage at pre-stimulation with the maximum response to stimuli. These EAG experiments were performed between 2013 and 2016.
Response to Linalool
On the morning of the behavioral experiment, the butterflies were moved from the rearing cage to another cage and placed without food. The experiments started at 15:00. After the experiment was complete, butterflies were returned to the original rearing cage. The electrophysiological experiments were performed following Inoue et al. (2019). Butterfly specimens of P. machaon, P. xuthus, P. maackii, P. protenor, and P. memnon were used in this experiment. The butterflies (except P. memnon females) were captured in 2018 on August 30 in Wakayama.
Results
Behavioral Experiments
The results of the behavioral experiments are provided in Fig. 6. The volatiles released by Citrus species, their preferred host plants, strongly induced the oviposition behavior of female P. xuthus, P. protenor, and P. memnon. However, some individuals of P. xuthus and P. protenor responded to Z. ailanthoides comparably or more robust than their response to Citrus spp. The graphic patterns of the preference behavior by female P. maackii and P. bianor (species that tend to lay eggs on plants other than Citrus) were different from those of P. xuthus, P. protenor and P. memnon. Females P. maackii and P. bianor preferred any other plant to the Citrus spp. The preference pattern of P. helenus was similar to P. bianor, although we obtained limited results using P. helenus. Although the preference pattern of P. macilentus also resembled the patterns of P. xuthus, P. protenor, and P. Memnon, the response of P. macilentus to O. japonica was more significant than the responses of P. xuthus, P. protenor, and P. memnon. P. machaon showed a unique pattern.
Electrophysiology
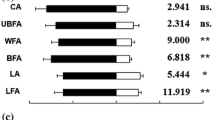
An example of the antennal responses (EAG) of P. protenor to leaf volatile substances released from Z. ailanthoides is presented in Fig. 7. The results of electrophysiological experiment for each female Papilio species are provided in Fig. 8. In general, P. xuthus, P. helenus, P. protenor, and P. memnon responded to volatile substances released from non-Citrus plants more strongly than to volatiles from Citrus spp. However, some individuals of P. xuthus and P. protenor, had a lesser response to Z. ailanthoides than Citrus spp. The response patterns of P. maackii, P. bianor, P. macilentus, and P. machaon (who preferably lay eggs on non-Citrus plants) were different from the other four species. The interspecies variations were high.
Results of the female EAG experiments with volatile substances from five plants. The vertical axis presents the magnitude of the relative response of the EAG. The graphs follow the description provided in Fig. 5. The red line in Fig. 7-g utilized data obtained using the antennae from a female P. macilentus
The results of the EAG responses by the males were identical to the conspecific females (Fig. 9). In both sexes, the EAG responses to the volatile substances of popular plants (highest scores) in the behavioral experiments were small compared with the low-scoring plants.
Results of the male EAG experiments with volatile substances from five plants. The graphs follow the description provided in Fig. 5
It was difficult to compare the behavioral and electrophysiological data using common statistical analyses like ANOVA, because 1: the number of data (n) was insufficient in some species, 2: the obtained data was not normally distributed, 3: the scores of each plant maybe not perfectly independent, with the possibility that the scores were affected by the trial order (see Figs. 5, 7, and 8). Thus, we provide the raw data without any statistical analyses.
Response to Linalool
The results of the Linalool experiments are provided in Table 2. The butterflies responded to Linalool in one or both experiments (behavioral and/or electrophysiological).
Discussion
We performed behavioral and electrophysiological examinations using eight Japanese Papilio species with four Rutaceae and one Apiaceae plant. The electrophysiological experiments showed that the antennal olfactory responses to volatile substances from the non-oviposition plants were generally greater than those to oviposition plants. Therefore, turning Fig. 6 (behavioral responses) upside down is a close replica of Figs. 8 and 9 (electrophysiological responses). P. cresphontes demonstrated a similar response in an EAG analysis (Fadamiro et al. 2010). Roessingh et al. (1991) showed the responses of the foreleg tarsal taste sensilla of P. polyxenes to non-hostplant leaf extracts were more significant than those to hostplant leaf extracts. Inoue (2006) reported that all species of Japanese Papilio butterflies have the same foreleg tarsal taste sensilla responses as those of P. polyxenes. Niki and Kanzaki (1995) also found the same tendency in P. xuthus, and Honda et al (2011) also found the same tendency in P. maackii and P. protenor. Therefore, we concluded that these tendencies apply to all Papilionini species. The similarity in the response to olfactory and taste stimuli strongly suggests that both stimuli are processed similarly by the central nervous system.
The EAG responses of the males of both the Citrus-preferring species and non-Citrus preferring species were nearly identical to those of the conspecific females, except P. protenor, P. bianor and P. machaon. The strength of EAG response of P. cresphontes to the plant volatile substances altered unexpectedly. The dose of 10 μg, Z. clava-herculis induced the largest response from both sexes. In contrast, at a dose of 100 μg, Ptelea induced largest response from females and Sassafras induced largest response from the males. At a dose of 1000 μg, Sassafras induced the largest response from both sexes (Fadamiro et al. 2010). We were unable to record the dose response curve. We suggest altering the volume of source leaves varies the volatiles providing different results with each experiment. The recently emerged females remain near the host plants from which they emerged. It is reasonable that the males prefer the same plant as the females because the males can easily find newly emerged conspecific females near their hostplants.
According to Inoue et al (2019), antennae of P. xuthus, P. maackii, and P. protenor showed no sexual dimorphism and are morphologically identical to one another with both sexes responding to ammonium volatiles and males play puddling behavior around ammonium volatiles in the field. Inside the experimental cage, unlike females, male butterflies do not perform the search flight. Therefore, the behavioral cage experiments were not effective with males. However, because the EAG responses to plant volatiles were almost identical between the sexes, the plant volatile responses are valid for both sexes. Conversely, Mozūraitis et al. (2016) stated that “volatiles released from foliar extract of host plants enhance the landing rates of gravid Polygonia c-album females, but do not stimulate oviposition.” These facts indicate that the role of volatiles in oviposition behavior is different between Papilionidae butterflies and Nymphalidae butterflies.
There was large individual variation in hostplant selection in our experiments, particularly in P. xuthus, P. protenor, P. bianor and P. machaon (Figs. 6 and 8). This result may indicate that there are genetic differences in herb selectivity by these species. Among these species, P. xuthus, P. protenor, and P. bianor have a wide hostplant selecting spectra. Hopkin's hypothesis (Hopkins 1917) states that butterflies are primed to prefer the hostplant species on which they fed as larvae. In our current experiments, larva of P. xuthus and P. protenor were reared on Z. ailanthoides and many emerged into females that preferred Citrus spp. as their oviposition plant. Therefore, Hopkin's hypothesis is not applicable to our results.
We previously assessed 10 Rutaceae and four Apiaceae volatile substances and we classified these plants into six groups based on the detected volatile components (Inoue et al 2023). We partially identified relationships between the plant species classification by the volatile components and the host plant preference by the butterflies, but in many cases, the individual differences between the butterflies were large, causing definite relationships hard to identify. These large variations may originate from genetic factors, and further investigations into its causes are required in future research.
The limited data available for P. helenus, P. bianor, and P. macilentus can be attributed to the high volume of discarded data from all the trails using these species when compared to the other five species. The high volume of discarded data may have originated from the inadequate conditions (probably the brightness condition of the experimental room or shortage of amount of leaves) of our current behavioral experimental room for P. helenus, P. bianor, and P. macilentus. In addition, females of these species were not easily captured from the wild, the number of specimens used in the experiment was limited.
Our results suggest Linalool may be important in the flower searching by Papilio butterflies. Especially in EAG experiments, all individuals responded to Linalool, instead in some individuals, they did not respond in behavioral examination (Table 2). There are many other flowers that Papilio butterflies sip, it is important to identify whether other flowers contain Linalool.
In the future, we would also like to attempted to make an inventory of the volatile substances of the host and related plants using Gas Chromatography with an Electroantennogram-Detecting system (GC-EAD).
References
Fadamiro H, Chen L, Akotsen-Mensah C, Setzer WN (2010) Antennal electrophysiological responses of the giant swallowtail butterfly, Papilio cresphontes, to the essential oils of Zanthoxylum clava-herculis and related plants. Chemoecology 20:25–33
Fukuda H, Hama E, Kasuya K, Takahashi A, Takahashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1982) Life history of butterflies in Japan 1. Hoikusha, Ôsaka. (in Japanese)
Honda K, Ômura H, Chachin M, Kawano S, Inoue TA (2011) Synergistic or antagonistic modulation of oviposition response of two swallowtail butterflies, Papilio maackii and P. protenor, to Phellodendron amurense by its constitutive prenylated flavonoid, phellamurin. J Chem Ecol 37:575–581
Hopkins AD (1917) A discussion of C. G. Hewitt’s paper on “Insect Behaviour.” J Econ Entomol 10:92–93
Inoue TA (2006) Morphology of foretarsal ventral surfaces of Japanese Papilio Butterflies and relations between these morphology, phylogeny and hostplant preferring hierarchy: Zool. Sci 23:169–189
Inoue TA (2013) Records of Papilio butterflies observed at Japan, Tsukuba, Ôwashi, 2011. JESUTIO 206:5–8 (in Japanese)
Inoue TA, Yukuhiro F, Hata T, Yamagami S, Yokohari F (2019) Ammonia as a puddling site-marshaling substance for Japanese Papilio butterflies. Chemoecology. https://doi.org/10.1007/s00049-019-00284-2
Inoue TA, Otani H, Niihara K, Fukuda T (2023) Inventory of leaf and flower odorants in plants associated with the life cycle of Japanese Papilio butterflies. Agric Biol Res J. https://doi.org/10.35248/0970-1907.23.39.441-448
Mozūraitis R, Radžiutė S, Apšegaitė V, Cravcenco A, Būda V, Nylin S (2016) Volatiles released from foliar extract of host plant enhance landing rates of gravid Polygonia c-album females, but do not stimulate oviposition. Entomol Exp Appl 158(3):275–283. https://doi.org/10.1111/eea.12405
Nihira I (2004) Hostplants of Japanese butterflies. Published by the author. (in Japanese)
Niki S, Kanzaki R (1995) Responses of tarsal contact chemoreceptors to oviposition stimulants in swallowtail butterfly, Papilio xuthus. Zool Sci 12(supple):101
Roessingh P, Städler E, Schöni R, Feeny P (1991) Tarsal contact chemoreceptors of the black swallowtail butterfly Papilio polyxenes: responses to phytochemicals from host- and non-host plants. Physiol Entomol 16:485–495
Acknowledgements
We thank the many people who informed us of Papilio butterfly observation sites and provided us with butterflies for the experiments (Inoue et al. 2023). The experimental design of the behavioral study on butterflies utilized the advice of Prof. Hisashi Ômura and Prof. Kei-ichi Honda, Hiroshima University, to whom we express our gratitude. Finally, we also give our special thanks to Editage staff who gave us great advice on this article.
Funding
This work was partially supported by JSPS KAKENHI (18K06393 to FT).
Author information
Authors and Affiliations
Contributions
Methodology: Takashi A. Inoue, Kinuko Niihara, Fumio Yokohari; Formal analysis and investigation: Takashi A. Inoue, Mami Suetake, Narumi Nishidzu, Fumio Yokohari; Writing – original draft preparation: Takashi A. Inoue, Kinuko Niihara; Writing – review and editing: Fumio Yokohar; Funding acquisition: Tatsuya Fukuda.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Takashi A. Inoue, Takashi A. Inoue, Mami Suetake, Narumi Nishidzu, and Fumio Yokohari. The first draft of the manuscript was written by Takashi A. Inoue and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
None
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inoue, T.A., Suetake, M., Nishidzu, N. et al. Behavioral and Electrophysiological Study on Eight Japanese Papilio Species with Five Hostplant Volatiles and Linalool. J Chem Ecol 49, 397–407 (2023). https://doi.org/10.1007/s10886-023-01433-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01433-2