Abstract
There has been a dearth of research elucidating the behavioral effect of microbially-produced volatile organic compounds on insects in postharvest agriculture. Demonstrating attraction to MVOC’s by stored product insects would provide an additional source of unique behaviorally-relevant stimuli to protect postharvest commodities at food facilities. Here, we assessed the behavioral response of a primary (Rhyzopertha dominica) and secondary (Tribolium castaneum) grain pest to bouquets of volatiles produced by whole wheat that were untempered, or tempered to 12%, 15%, or 19% grain moisture and incubated for 9, 18, or 27 days. We hypothesized that MVOC’s may be more important for the secondary feeder because they signal that otherwise unusable, intact grains have become susceptible by weakening of the bran. However, contrary to our expectations, we found that the primary feeder, R. dominica, but not T. castaneum was attracted to MVOC’s in a wind tunnel experiment, and in a release-recapture assay using commercial traps baited with grain treatments. Increasing grain moisture resulted in elevated grain damage detected by near-infrared spectroscopy and resulted in small but significant differences in the blend of volatiles emitted by treatments detected by gas chromatography coupled with mass spectrometry (GC–MS). In sequencing the microbial community on the grain, we found a diversity of fungi, suggesting that an assemblage was responsible for emissions. We conclude that R. dominica is attracted to a broader suite of MVOC’s than T. castaneum, and that our work highlights the importance of understanding insect-microbe interactions in the postharvest agricultural supply chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olfaction mediates many fundamental biological processes for insects. For example, volatile compounds may be used for foraging (Morrison et al. 2018a), mate-finding (Xu and Turlings 2018), development (Oi et al. 2015), dispersal (Rork and Renner 2018), as well as social group formation and cohesion (Buhl and Rogers 2016). These compounds may originate from a variety of sources, including plant volatiles (Xu and Turlings 2018), microbes (e.g., fungi, bacteria; Davis et al. 2013), conspecifics, and heterospecifics.
There have now been an increasing number of studies in a variety of systems exploring insect-microbe interactions, and how microbially-produced volatile organic compounds (MVOC’s) significantly alter ecological interactions (Bueno et al. 2020; Liu et al. 2016; Russell and Ashman 2019). For instance, it is known that MVOC’s may aggregate insects, attract individuals, promote mating or oviposition, elicit feeding, or act as strong repellents (reviewed in Davis et al. 2013). The behavioral response to the same microorganism or MVOC may vary by species or abiotic conditions (Hoftstetter et al. 2007). Further, MVOC’s may signal host suitability and may even indicate a specific timeframe for optimal conditions to insects (Beck and Vannette 2017). Davis and Landolt (2013) found that over 1,300 insects, representing 39 species, responded to three common fungal volatiles, 2-methyl-1-butanol, 3-methyl-1-butanol, and 2-phenylethanol, found in the headspace of the yeast-like fungus Aureobasidium pullulans on a spearmint farm. The authors concluded that the insects were not responding haphazardly, because of the low insect attraction to traps with competing volatiles from another species of fungus. In some cases, insects will respond similarly to microbial assemblages as to microbial monocultures (Rering et al. 2020). Thus, in the last decade, there has been an increasing amount of work documenting the ubiquity and importance of MVOC’s for insect behavioral responses (Beck et al. 2018; Davis et al. 2013; Ponce et al. 2021; Weisskopf et al. 2021).
In evolutionary terms, stored product insects have only relatively recently switched to feeding on agricultural goods (e.g., in the last 10,000 years bp). For most of their history, stored product insects were well-adapted for dispersing to, finding, and utilizing woody hosts (reviewed in Quellhorst et al. 2021) or animal caches of stored food (Jia et al. 2008). Animals typically store their food in damp conditions, often near the surface but underground or else in a concealed location (Hagstrum and Phillips 2017), which is an ideal environment for the growth of fungi and other microbes. Only about 60% of the caches were recovered by pine chipmunks in Nevada, for example (Kamil and Gould 2008), suggesting that at least 40% of their seed caches, but perhaps all of them, would be available for exploitation by stored product insects, depending on length of storage. Possible kairomones of that food source for stored product insects may be MVOC’s produced through colonization of and selective growth given the environmental conditions in animal caches by microbes (Morrison et al. 2021). Thus, there may be a conserved response by stored product insects to MVOC’s.
Indeed, attraction to MVOC’s by stored product insects has been observed in a variety of studies. For example, in a two-choice olfactometer, research demonstrated attraction of four species of stored product cucujid beetles to specific fungal volatiles, including 1-octen-3-one, 3-octanol, and 3-octanone (Pierce et al. 1991). Another study found that Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) were more strongly attracted to fungi associated with cotton seed than those found on certain grains (Ahmad et al. 2012). Similarly, uncrowded or moderately crowded Oryzaephilus surinamensis (L.) (Coleoptera: Cucujidae) and O. mercator (Fauvel) (Coleoptera: Cucujidae) were highly attracted to volatiles from Brewer’s yeast (Pierce et al. 1983). By contrast, Zunino et al. (2015) found that eight MVOC’s produced by Fusarium verticillioides were repellent to the maize weevil, Sitophilus zeamais (Motschulsky) (Coleoptera: Cucurlionidae). Furthermore, other work found that stored-product pscocids were also repelled by volatiles produced by Ulocladium botrytis and Eurotium amstelodami, fungal strains isolated from decaying paper and colony food sources, respectively (Green 2008). In a systematic review, Ponce et al. (2021) found stored product arthropods were both attracted to and repelled by microbial cues, depending on arthropod species, microbial taxon, and other factors. Thus, it seems clear that MVOC’s are important for mediating behavioral responses for a range of stored product insects. While it may be impossible to work with sterile grain at food facilities, there has been an enormous amount of detail paid to hygiene in every process, including minimizing the occurrence of off-odor grain from microbial growth by creating conditions that are hostile for microbial proliferation. Thus, in modern food facilities, there is likely overall a dearth of MVOC’s because many managers of food facilities attempt to keep production areas well-sanitized (Morrison et al. 2019a, b; Morrison et al. 2021). As a result, MVOC’s represent at once both a potentially highly potent source of volatiles, and also unique cues in the post-harvest environment that is otherwise saturated with food stimuli. This potentially makes MVOC’s ideal to deploy against stored product insects to improve behaviorally-based monitoring and management tactics in food facilities. In particular, these cues may be useful in perimeter-based, attract-and-kill systems (Morrison et al. 2019a, b), push–pull systems that include a repellent (Pickett et al. 2014), or through the use of interception and spillage traps that include additional semiochemicals (Wilkins et al. 2021).
The microbial community for commodities in the field may be significantly different from that found after harvest during storage with an attendant change in key volatiles. For example, in sampling wheat heads in the field, the most common genera of fungi were Alternaria, Cladosporium, Sporobolomyces, Blumeria, and Cryptoccus, which together accounted for 83% of the species (Hertz et al. 2016). By contrast, the most abundant genera in storage on durum wheat were Alternaria, Fusarium, Penicillium, Aspergillus, Rhizopus, and Mucor, which together accounted for 96% of the species (Belkacem-Hanfi et al. 2013). While a systematic review found that eight-carbon compounds were common fungal volatiles in headspace (Ponce et al. 2021), no eight-carbon compounds were found emitted from a winter wheat field (Bachy et al. 2020), suggesting that in some cases the volatile composition in the field may vary greatly from microbial volatiles in storage. Thus, the food storage environment is a unique environment with its own biota and ecological dynamics worthy of investigation in its own right.
Tribolium castaneum and Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) are two important species in the food storage environment, and represent two different ends of a life history continuum for many variables, which may shed light on how life history shapes the use of fungal volatiles in foraging. While the former is a secondary pest, attacking finished or already damaged goods, R. dominica is a primary pest, seeking out intact grain kernels in which to oviposit and develop (Perišic et al. 2017). In terms of movement, T. castaneum is a strong walker but weak flier, while the opposite is true of R. dominica (Morrison et al. 2018b). Whereas T. castaneum is typically found associated with food facilities (Ridley et al. 2011), R. dominica can be found in high abundance elsewhere in the landscape far from food facilities (Mahroof et al. 2010). Tribolium castaneum has been described as facultatively frugivorous, with demonstrated attraction at small spatial scales to fungal volatiles from linted cotton (Ahmad et al. 2013), whereas there has been no study yet on the behavioral response of R. dominica to MVOC’s.
It is well-known that grain moisture affects a variety of parameters important for postharvest commodity protection. For example, most obviously, grain moisture may readily affect commodity quality (reviewed in Ziegler et al. 2021), and this is a parameter that is strictly monitored in food facilities. Generally, food facilities are xeric (very dry) environments, and some stored product insects have adapted to these conditions with the ability to make their own metabolic water in place of taking in water externally (Murdock et al. 2012). However, hotspots of insect infestation result in excess moisture in the commodity, which is absorbed by the grain, and may promote the growth of fungi and other microbes present on the commodity (Dunkel 1988). In addition, fungal growth in a xeric environment may be slow, but inexorable, and storage over increasing periods of time may lead to microbial spoilage. As a result, both grain moisture and storage interval are important determinants of microbial activity on grain.
The goal of the study was to evaluate the behavioral responses of T. castaneum and R. dominica to the MVOC’s produced by stored grain. We hypothesized that MVOC’s may be more important for the secondary feeder, T. castaneum, because MVOC’s may signal that otherwise unusable intact grains have become susceptible by weakening of the kernel through fungal activity. This may allow the secondary pest to more easily penetrate the bran of a kernel. We investigated the effects of grain moisture and incubation period on insect behavioral responses, and we also characterized changes in the volatile emissions caused by these variables.
Methods and Materials
Experimental Insects and Colony Maintenance
Tribolium castaneum were from a colony derived from a field population from central Kansas in 2012 and were reared on a mixture of 95% unbleached, organic flour and 5% brewer’s yeast. Rhyzopertha dominica were from a colony derived from a field population collected outside a mill in central Kansas in 2012 and were reared on organic wheat. Both colonies were maintained in an environmental chamber set at constant 27.5 °C, 60% RH, and 14:10 h (L:D) photoperiod.
For each assay, 4–8 wk-old adult were used. All beetles (in a 1:1 male:female sex ratio) for the assays were pulled from the colonies on the same day as they were to be used and held in individual plastic containers (30 mL, Maryland Plastics, Federalsburg, MD, USA).
Treatment Preparation
To induce microbial growth for volatile and behavioral assays, microbial growth was promoted by tempering organic, hard winter wheat all obtained from a single farmer in Kansas to specific moistures through the addition of reverse osmosis, deionized water. To assess the baseline moisture level of the source grain, a grain moisture analyzer was used (DICKEY-john, GAC2100, Auburn, Illinois, USA). Source and control grain contained a moisture level of 10.8%. Every 7–9 d, from 21 May to 25 Jul 2018, two replicates of 200 g of organic wheat were tempered up to 12%, 15%, or 19% grain moisture levels by pipetting the appropriate amount of water, and thoroughly mixing grain. This represents the range of reasonable grain moisture levels for most commodities, as well as one just outside of that range (e.g. 19%), which mimics an insect hotspot or other control failure. Ambient fungal and microbial spores located on the grain itself formed the basis for microbial growth on the tempered grain. After tempering, batches of grain were incubated in an environmental chamber (30 °C, 60% RH, 14:10 L:D; Percival Instruments, Dallas County, IA, USA) for 9, 18, or 27 (± 2) d to promote microbial growth. As a negative control, we compared the grain moisture treatments to clean grain without the addition of extra water (i.e. untempered). Finally, for the maximally damaged grain by microbes mentioned for the near-infrared spectroscopy, grain was prepared as above and tempered to 19% grain moisture; the same amount of water was then added to the grain on a weekly basis for six weeks.
Near-Infrared Spectroscopy of Grain
A multispectral sorting device was used to assess the proportion of grain kernels in treatments that were infested with fungi and other microbes, which manifested in color or reflectance changes (Pearson et al. 2013). Prior work has found that this sorter can correctly identify 90% of grain kernels damaged with the fungal disease, Fusarium head blight, from undamaged kernels (Pearson et al. 2013). The sorter used three visible and three near-infrared light-emitting diodes with peak emission wavelengths of 470 nm, 527 nm, 624 nm, 850 nm, 940 nm, and 1070 nm by rapidly blinking each LED (~ 12 kHz) in quick succession, and measuring the reflected light from the wheat kernels using a miniature 16 mm lens (V-4316-2.0, Marshall Electronics, Inc., CA, USA) as kernels dropped off a feeder chute at a 45° angle with a speed of 1.5 m/s. The device employed a microcontroller (ATmega328P, Atmel Corp., San Jose, CA) that directs the LED pulses, digitizes the analog signal from a photodiode (PDB-C171SM, Advanced Photonix, Inc., Ann Arbor, MI, USA), performs signal processing, and applies classifications. The device sorted grain at 20 kernels/s through the activation of an air valve that diverted the wheat kernels based on a prior calibration. Calibration of the machine occurred by training the machine to recognize damaged grain by using 200 fungal-damaged kernels and 200 undamaged kernels. Prior to data collection, we evaluated the accuracy of the sorter by calibrating the device based on training with the 12%, 15%, and 19% grain moisture incubated at 27 d, or for maximally infested grain (as described above) in comparison to clean grain held at 10.8% moisture in each case. The 19% grain moisture calibration was used for data collection based on its high accuracy (see results below), to obtain a conservative estimate of microbial damage, and to ensure consistency with incubation methodology compared to all other treatments. To evaluate damage, 100 g of grain, approximately 1,900 individual kernels, was sorted for each combination of grain moisture and incubation period, including the undamaged grain (control).
Wind Tunnel Assay
A wind tunnel assay was used to better understand the taxis and attraction of T. castaneum and R. dominica to fungal volatiles from the grain. In the wind tunnel, air was pushed through a turbine (diameter: 36.5 cm), an activated carbon filter to purify the air, and two slatted-metal sieves (73 × 85 cm) to create a laminar airflow, with an average airspeed of 0.38 m/s. A total of 20 g of each odor treatment described above was placed 13.5 cm upwind of the stimulus edge of the test arena and 5 cm from the last sieve. Each odor treatment was held in a plastic petri dish (100 × 15 mm D:H). The adults were placed individually in the release point at the center of an arena consisting of a 21.6 × 27.9 cm sheet of paper. For all replicates, each adult remained walking in the wind tunnel. In each round of sampling, mixed-sex adult beetles were collected from their colonies using a sieve (No. 25, W.S Tyler Inc., Mentor, Ohio). Each adult was given a maximum of 2 min to respond, and the edge of the arena on which the adult exited was recorded as either the stimulus edge (upwind edge of the arena), or a non-stimulus edge (one of the other three edges) (Fig. 1A). In addition, the time to decision was recorded for each individual. Adults that did not respond within the timeframe were excluded from the final statistical analysis. Each adult was considered a replicate and was never used more than once. Odor flow from the petri dish to the release arena was confirmed with a smoke test. In total, there were N = 60 replicates per each species and treatment combination of grain moisture and incubation interval.
Release-Recapture Trapping Assay
To assess how fungal volatiles may improve capture of T. castaneum and R. dominica adults in monitoring traps, a trapping assay was utilized (Fig. 1B). In each replicate, 20 mixed-sex adults were settled on clean cardboard slats (8 × 8 cm) and then placed in one corner of a plastic bin (86.3 × 30.5 × 39.4 cm L:H:W). In the opposite corner, diagonally across from the release point in the bin, a pitfall trap (Dome Trap™, Trécé, Inc., Adair, OK, USA; Campbell 2012) was deployed. The base was rough along the ascending edges to create traction for the beetles; and the crest was concave and smooth to prevent subsequent escapes. Semiochemical stimuli were placed within the concave portion of the trap. Each trap contained either no grain (negative control), or 5 g of one of the treatments: untempered grain (positive control), grain tempered to either 12%, 15%, or 19% grain moisture, or 950 µl of Trécé- produced Storgard™ Oil (Trécé, Inc.), a known semiochemical kairomone and food cue, which is a standard lure used in commercial facilities (Campbell 2012) and acted as the positive control. All grain in this study was either incubated for 9 d, 18 d, or 27 d, and trapping assays were initiated on the same days as the wind tunnel assays described above. The bins were located in a large (5 × 6 × 2 m, L:W:H) walk-in environmental chamber (Percival Instruments, Dallas County, IA, USA) under constant conditions (25 °C, 65% RH, 14:10 L:D). The adults were given 24 h to respond to the stimuli. Afterwards, the traps were collected, the adults were sieved (No. 10, W.S Tyler Inc., Mentor, OH), and the number of beetles in each trap was recorded. On a given round of release, two replicates of each treatment listed above were performed simultaneously, for a total of 12 bins set up containing 240 adults total. In total, there were N = 8 replicates per species and combination of treatment and incubation, including the controls.
Volatile Collection
Headspace collection was performed in order to determine the volatiles released by the various moisture treatments (no grain as a negative control, or 20 g of untempered grain control, or grain tempered to 12%, 15%, or 19%) and incubated at different intervals (9 d, 18 d, 27 d). First, central air was scrubbed using an activated charcoal filter, then pushed through the remaining apparatus. The airflow was restricted to 1 L/min using a flow meter (Volatile Collection Systems, Gainesville, FL, USA) placed directly prior to the sample collection in headspace chambers (10.2 × 12.7 cm D:H) with an inlet for air and an outlet for a volatile collection trap (VCT). The headspace volatiles from the grain were collected for 3 h onto a VCT consisting of a drip tip borosilicate glass tube packed with 20 mg of absorbent Porapak-Q™ (Volatile Collection Systems, Gainesville, FL, USA) to adsorb volatiles with a stainless steel screen (No. 316) on one side, and held in place with a borosilicate glass wool plug followed by a PTFE Teflon compression seal. The volatiles on the traps were eluted with 300 µl of dichloromethane (Millipore, Billerica, MA, USA) by pushing the solvent through with inert nitrogen gas into 2-mL glass vials with caps containing Teflon-lined septa and then stored at −20 °C until analysis. Volatile collection traps were reused after they were washed three times with 700 µl of dichloromethane. Prior to analysis, tetradecane (190.5 ng, 99% purity, GC analytical grade, Millipore, Billerica, MA, USA) was added as an internal standard.. A total of N = 4–6 replicate collections were done for each combination of moisture and incubation treatment.
Gas Chromatography Coupled with Mass Spectrometry
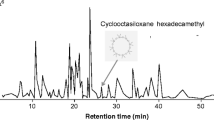
All headspace collection sample extracts were run on an Agilent 7890B gas chromatograph (GC) (Agilent Technologies, Inc., Santa Clara, CA, USA). equiped with an Agilent Durabond HP-5 column (30 m length, 0.250 mm diameter and 0.25 μm film thickness) with helium as carrier gas at a constant 1.2 mL/min flow and 40 cm/s velocity, which was coupled with a single-quadrupole Agilent 5997B mass spectrometer (MS). Samples were injected with an autosampler in split mode split (15:1 ratio with a split flow rate of 18 ml/min).The compounds were separated by auto-injecting 1 μl of each sample in split mode (250 °C) with flow was split in a 15:1 ratio with a split flow rate of 18 ml/min. The oven temperature was programmed at 40 °C for 1 min followed by 10 °C/min increase to 300 °C and then held for 26.5 min. After a solvent delay of 3 min, mass ranges between 50 and 550 atomic mass units were scanned. Compounds were tentatively identified by comparison of spectral data with those from the NIST 17 library and by GC retention index (Adams 2009). Compound peak areas relative to that of the internal standard were used to calculate the emission rates (ng/g of grain/h of collection).
Microbial Community Characterization
To characterize the microbial community on the grain, the microbes were first cultured on potato dextrose agarose (PDA) petri dishes (100 × 10 mm). This was performed by subsampling 5 g of grain that had been incubated for 27 d at 18% grain moisture. Each subsample was thoroughly agitated for 1 min, then four wheat kernels were placed in one of each of the quadrants of a petri dish with PDA in a biosafety cabinet (Labconco, Kansas City, MO, USA). This was done for three separate replicate dates of grain incubation and tempering. Thus, a total of 12 wheat kernels were initially cultured for 10 d in an incubator (IMC18, Heratherm, ThermoScientific, Waltham, MA, USA) at room temperature (approx 23 °C). For each dish, every unique microbial morphotype was streaked onto a new PDA petri dish, and allowed to incubate for a further 10 d under the same conditions. At the end, 200 mg of fungal material was excised and used for DNA isolation and sequencing below. A total of N = 25 excisions was taken.
Microbial Community DNA Isolation and Sequencing
Excised fungi were placed into 1.5-ml microcentrifuge tubes and DNA was extracted using the Quick-DNA Fecal/Soil Microbe Miniprep Kit (D6010, Zymo Research, Irvine, CA, USA). The ITS region of the fungal DNA was amplified using polymerase chain reaction (PCR), using primers that consisted of ITS4 5’-TCCTCCGCTTATTGATATGC-3ʹ and ITS5 5ʹ-GGAAGTAAAAGTCGTAACAAGG-3ʹ (White et al. 1990). In each reaction, 1 μl of each of extracted DNA and primer (10 μM), 9.5 μl of nuclease free water, and 12.5 μl of master mix containing 50 units/ml of Taq DNA polymerase (Hot Start Taq 2X Master Mix, Promega, Madison, WI, USA) were combined in a proprietary reaction buffer (pH 8.5). Briefly, the PCR program consisted of 2 min of initial denaturation at 95 °C, followed by 30 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1.5 min. Afterwards, a final extension was performed at 72 °C for 5 min, then PCR products were held chilled at 4 °C. To clean up the samples, 5 μl of PCR products were mixed with 2 μl of ExoSAP-it (ThermoFisher Scientific, Waltham, MA, USA), then placed in the thermal cycler at 37 °C for 15 min, and ramped to 80 °C for a further 15 min. Finally, the amplicons were sent for bidirectional sequencing on an ABI 3730XL instrument (Eurofins Scientific, Brussels, Belgium), and the resulting sequences were quality-filtered and aligned using Sequencher (v. 5.4.6, Gene Codes, Ann Arbor, MI, USA). The consensus sequences were searched against NCBI’s nucleotide database (nt) using the BLASTn algorithm (Altschul et al. 1997). In order to circumvent taxonomic misassignments, the consensus sequences were also checked against Michigan State’s Ribosomal Database Project (RDP) that searches the UNITE Database (Wang et al. 2007). The consensus sequences were submitted to GenBank and under the accession numbers MT883436–MT883460.
Sensory Panel Evaluation of Grain
In order to confirm that the volatiles coming off of the grain were indeed of microbial in origin and not simply grain volatiles that have also been ascribed a dual purpose as MVOC’s from the literature, an informal sensory panel was convened from three volunteer technicians at the USDA Center for Grain and Animal Health Research. Each volunteer was familiar with unadulterated and spoiled grain from extensive experience handling insect colonies on grain. Each volunteer was given the opportunity to smell the bouquet of N = 4 replicates of each treatment combination of incubation period (9d, 18 d, 27 d) and grain moisture (ctrl, 12%, 15%, 18%) listed above and was asked to report the odor as “grain-like”, “spoiled”, or “other”. Exactly 5 g of each treatment was placed in a 3-oz capacity plastic container, were covered with lids, and then randomly assigned a location on tray with the identity of the treatment known only to an impartial third observer not participating in the study. Volunteers then sequentially sniffed each treatment, leaving 1 min between each sniff in order to allow a recovery time to cleanse the palette prior to additional tests. On a given day, a single person smelled 16 samples.
Statistical Analysis
Differences in fungal damage from near-infrared spectroscopy were analyzed by comparing the proportion of damaged grain (by weight) of 100 g among grain moisture treatments, and within a grain moisture treatment among incubation intervals using χ2-tests relative to the null hypothesis that damage was equal between treatments. A Bonferroni correction to the α-threshold was used to control for the family-wise error rate.
To analyze the data from the wind tunnel assay, a generalized linear model based on a binomial distribution was employed. In particular, adult beetles that exited on the stimulus (coded as 1) or non-stimulus edge (coded as 0) of the arena was used as the response variable. The two explanatory variables included in the model were the grain moisture level (control, 12%, 15%, or 19%) and incubation interval (9 d, 18 d, or 29 d), as well as their interaction. The model was evaluated for overdispersion, which was not found to be an issue. Log-likelihood tests for significance were conducted based on a χ2-distribution. Upon a significant result from the model, generalized linear model multiple comparisons was conducted using the function glht in the multcomp R package (Hothorn 2017) which implements Tukey’s HSD for generalized linear models. For this and all other analyses, R v.4.0.3 was used (R Core Team 2021), and α = 0.05, except where otherwise noted.
Differences in the time (seconds) to decision for the wind tunnel were analyzed with a 2-way analysis of variance (ANOVA). The model structure was of the same form as the one in the preceding paragraph. After inspection of residuals, the data conformed to the assumptions of normality and homogenous variances, and thus no transformation was necessary. After a significant result from the overall model, Tukey’s HSD was used for pairwise comparisons.
For the trapping assay, data were analyzed with a two-way ANOVA, using the number of adult T. castaneum and R. dominica recaptured in traps as response variables. The two explanatory variables included in the model were the lure treatment (no grain, untempered grain, grain tempered to 12%, 15%, or 19%, or Trécé Storgard Oil) and grain incubation interval (9 d, 18 d, or 27 d), as well as their interaction. The data conformed to the assumptions of normality and homogenous variances, and thus no transformation was necessary. After a significant result from the overall model, Tukey’s HSD was used for pairwise comparisons.
To analyze the volatiles collected from different grain moisture and incubation treatments, raw peak areas were extracted from the gas chromatograms using MSD ChemStation v2.00 software (Agilent Technologies, Inc., Santa Clara, CA, USA). Compounds were aligned between representative samples from each treatment based on retention time and quantified using the internal standard as above. Background volatiles found in the negative control without grain were discarded from the other samples, because these represent transient background volatiles in the general vicinity of headspace collection but are not informative of differences among the treatments. In addition, headspace compounds found in only two samples were discarded, because these represent transient emissions not representative of differences among treatments. Using the emissions of individual volatiles, Bray–Curtis dissimilarities were calculated in a pairwise fashion between each headspace sample, and non-metric multi-dimensional scaling (NMDS) was used to visualize the differences in volatile emissions among treatments. A total of N = 1000 permutations was used for the ordination procedure. Stress values for the NMDS procedure were < 0.1, indicating that good interpretation was possible. An analysis of similarity (ANOSIM) was used to determine significant differences for headspace volatiles among levels within grain moisture (negative control, positive control, 12%, 15%, 19%), and by incubation interval (9 d, 18 d, 27 d). A total of n = 1000 permutations was performed for the test. For all multivariate statistics, the vegan package was used in R (Oksanen et al. 2019).
To analyze the data from the sensory panel evaluation, a generalized linear model based on a binomial model was used. The response variable included whether samples were spoiled (yes/no), with incubation period, grain moisture, and their interaction used as fixed explanatory variables. Overdispersion was checked and found not to be an issue with the model. Multiple comparisons employed chi-squared tests with Bonferroni correction to the alpha-threshold.
Result
Near-Infrared Spectroscopy
Overall, the sorter was able to accurately sort 95% of the sound kernels from the damaged grain and had an error rate of 13% for sorting the damaged grain from the sound kernels. In ascertaining fungal growth, the percentage of damaged kernels present differed significantly among all grain moisture treatments (pairwise χ2 – tests, P < 0.05; Fig. 2). In general, as the moisture increased in the grain from the control to 19%, the percent of damaged grains nearly doubled at each level. The incubation intervals did not significantly affect the amount of damaged grain (χ2-tests, Bonferroni correction; Fig. 2). Overall, the lowest percent of damaged grain was in the untempered control grain, which was almost 10 times less than the 19% grain moisture treatment.
The percent of damaged wheat grain kernels from 100 g per incubation interval and treatment based on sorting by a near-infrared device. Lower case letters are comparisons within a moisture treatment, while upper case letters are comparisons among grain moisture treatments. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction)
Wind Tunnel Assay
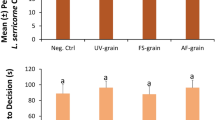
Grain moisture did not significantly affect the proportion of T. castaneum adults choosing the upwind stimulus edge of the arena (χ2 = 4.33; df = 3; P = 0.63; Fig. 3). The total percentage of adults choosing the stimulus side ranged from 28% to 37% in the various grain moisture treatments. In addition, we found that there was no significant difference in the proportion of T. castaneum choosing the stimulus edge of the arena at different incubation intervals (χ2 = 1.59; df = 2; P = 0.21; Fig. 3), which ranged from 27% to 44%. The interaction between grain moisture and incubation time also had no significant effect on the behavior of adult beetles (χ2 = 6.32; df = 6; P = 0.39). There was only an 18.7% difference between the treatments eliciting the lowest and highest percentages of adults that chose the stimulus edge. Likewise, neither the grain moisture (F = 0.09; df = 3, 698; P = 0.96) nor the incubation interval (F = 0.13; df = 2, 698; P = 0.72) significantly affected T. castaneum adult decision time. There was also no significant grain moisture by incubation interval interaction on the time it took T. castaneum adults to reach an edge (F = 0.49; df = 6, 698; P = 0.69).
The percent of A Tribolium castaneum (N = 60 replicates per bar) and B Rhyzopertha dominica (N = 60 replicates per bar) adults that chose the stimulus edge in response to grain moisture treatments and incubation intervals in a wind tunnel assay. Lower case letters are comparisons within a moisture treatment. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction). No letters are displayed for T. castaneum, because statistical tests for the overall model were not significant
However, we found that there was a significant difference in the attraction of R. dominica to the various incubation levels (χ2 = 5.86; df = 2; P < 0.05; Fig. 3), with 1.6-fold greater attraction to grain incubated for 27 d than 9 d. In contrast to T. castaneum, we found that the grain moisture treatments had a significant effect on the percentage of R. dominica choosing the stimulus edge (χ2 = 15.1; df = 3; P < 0.01; Fig. 3). The total percentage of adults that chose the stimulus edge was two-fold greater in the 19% grain moisture treatment compared to the grain tempered to 12%. There was no significant interaction found between grain moisture treatments and incubation intervals (χ2 = 3.65; df = 3; P = 0.30; Fig. 3).
However, the grain moisture did not significantly affect the time required for R. dominica adults to make decisions (F = 0.93; df = 3, 466; P = 0.43). In addition, the incubation interval did not significantly affect adult decision time (F = 3.04; df = 2, 466; P = 0.08), with the time to decision ranging from 58 to 63 s. There was no significant interaction between grain moisture and incubation interval on the time it took R. dominica adults to make decisions (F = 0.99; df = 6, 466; P = 0.40).
Release-Recapture Trapping Assay
In the release-recapture assay, across all treatments and incubation levels, 42.4% of T. castaneum adults released were recaptured, indicating a sufficient sample size to form conclusions. We found that there was no significant difference between grain moisture treatments on the recapture of T. castaneum adults (F = 0.43; df = 5; P = 0.83; Fig. 4). The largest difference in the percentage of adults recaptured in an incubation interval was 38.1% between the negative control and the commercial TSO (Storgard) lures at 27d. The number of adults recaptured was not significantly different among incubation intervals (F = 0.063; df = 2; P = 0.80; Fig. 4), which ranged between 27% and 44% across moisture treatments. Finally, the interaction between grain moisture and incubation interval did not have a significant effect on the recapture of adults (F = 0.69; df = 5; P = 0.63; Fig. 4).
The percent (± SE) of Tribolium castaneum (N = 8 replicates per bar) and Rhyzopertha dominica (N = 8 replicates per bar) adults recaptured in pitfall traps which contained various semiochemical treatments. Empty: unbaited pitfall trap, Ctrl: positive control of untempered grain, TSO: Trécé Storgard oil, 12, 15, 19: grain moisture treatments. For both species, the interaction between semiochemical treatments and incubation times was not significant. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05). No letters are displayed for T. castaneum, because statistical tests for the overall model were not significant
Similar to T. castaneum, across all treatment combinations, 28% of all released R. dominica adults were recaptured in the pitfall traps, indicating a sufficient sample size to develop conclusions. However, in contrast to T. castaneum, the semiochemical lures had a significant effect on the capture of R. dominica adults compared to empty traps (F = 4.31; df = 5; P = 0.001; Fig. 4). Rhyzopertha dominica showed elevated capture in pitfall traps with moderate fungal growth in grain tempered to 12% (Tukey HSD, Fig. 4). The total percentage of adults captured in the traps with 12% grain moisture was 3.6 times greater than empty traps. The incubation interval did not significantly affect the number of R. dominica adults in traps (F = 0.22; df = 2; P = 0.64; Fig. 4).
Volatile Collections
The GC–MS analyses of volatiles collected showed that, while there was significant overlap in volatile emissions by treatments, there was some significant differentiation. For example, grain moisture had a small but significant impact on the composition of volatiles emitted from the treatments (R = 0.116; Perm = 1000; P < 0.001; Fig. 5). However, there was no significant difference among the volatiles emitted from grain at different incubation intervals (R = −0.033; Perm = 1000; P = 0.93; Fig. 5). While the headspace from the different incubation intervals were equally complex (χ2 = 0.09, df = 2, P = 0.95), each with about 15 compounds, the grain tempered to 19% moisture was more complex (χ2 = 4.15, df = 2, P < 0.05), with 1.6-fold more compounds than the positive control consisting of unmanipulated grain (Supplementary Table 1). Tempered grain appears to be enriched in 2-methyl- and 3-methyl-1-butanol compared to the positive control grain as well as other assorted alkane and alkene alcohols (Supplementary Table 1).
Non-metric multidimensional scaling (NMDS) ordination of headspace volatiles from grain samples that were not tempered (positive control, grain moisture ≈ 10.8%), or tempered to 12%, 15%, or 19% grain moisture and incubated for 9 d, 18 d, or 27 d (N = 4–6 replicates per treatment combination). Shading of samples (circles) is based on A Grain moisture, B Incubation period, or C Their interaction. Ordination is based on pairwise Bray–Curtis similarities calculated among samples. Calculated stress is < 0.06 after n = 1000 permutations, indicating interpretation is possible
Microbial Community Characterization
In all, 25 samples were sequenced. Most of the fungi identified belonged to the genus Alternaria (68%), followed by Aspergillus (16%), Diplodia (8%), Penicillium (4%), and Epicoccum (4%) (Table 1). In total, nine species were identified from each of these genera, with the most species rich genus being Alternaria.
Sensory Panel Evaluation
Both the grain moisture (χ2 = 7.82; df = 3; P < 0.05) and incubation period (χ2 = 6.70; df = 2; P < 0.05) had a significant effect on the percentage of samples perceived as originating from a microbial source (Fig. 6). However, the interaction between the two factors did not significantly affect perception of microbial contamination (χ2 = 8.37; df = 6; P = 0.21). Other unprompted comments by the volunteers to describe the microbially-contaminated grain included: “musty”, “malodorous”, “moldy”, “sour”, “spoiled”, and “pungent”. Unprompted comments describing volatiles primarily from the unspoiled grain included: “grain-like”, “moist”, and “wet grain”.
Result of the sensory panel evaluation conducted by three technical personnel at USDA Center for Grain and Animal Health Research confirming source of volatiles as originating from microbial contamination (blue bars) or grain (orange bars). Each bar represents the overall percentage of N = 36 (top panel) or N = 48 (bottom panel) independent assessments conducted by three volunteers in a series of four replicates. Capitalized letters represent comparisons among grain-odor bars, while lower case letters represent comparisons among microbial odor bars. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction)
Discussion
In this study, we have demonstrated that the primary pest, R. dominica, is attracted to MVOC’s found in tempered and incubated grain samples, both in the wind tunnel and in a release-recapture experiment. The sensory panel confirmed that perception of microbial volatiles was highest among the treatments found to be most attractive to R. dominica. This was counter to our original hypothesis where we thought fungal cues may be more important to the secondary pest, T. castaneum. To our knowledge, this is the first study to document attraction of R. dominica to MVOC’s. Previous work reported that the presence of other stimuli such as aggregation pheromones (Dissanayaka et al. 2020; Edde et al. 2005; Leos-Martinez et al. 1987) or presence of conspecifics (Cordeiro et al. 2019) can affect the orientation of R. dominica. Furthermore, both sexes of R. dominica respond to plant volatiles, with the volatiles from wheat seeds being the most preferred (Edde and Phillips 2006). Additionally, when ethanol was added to the R. dominica aggregation pheromone (dominicalures 1 & 2) in funnel traps, there was a significant increase in field captures, but no increase was observed when a variety of essential oils or green leaf volatiles were combined with the pheromone (Edde et al. 2011). Bashir et al. (2001) found a synergistic attraction by both sexes of R. dominica to maize volatiles and conspecific cues. By contrast, Nguyen et al. (2008) found no evidence that walking R. dominica use cereal volatiles to locate grain. While the interactions between R. dominica with plant volatiles, grain volatiles, and conspecific cues has been extensively explored, its interaction with the microbial community has been neglected.
Contrary to our initial hypothesis, the secondary feeder, T. castaneum, did not seem to use microbial cues, while R. dominica did in fact use them and apparently to a much more generalized extent, given the diverse assemblage of microbes that may have been present on the grain. One potential evolutionary rationale for the use of microbial cues by R. dominica is in its historical use of woody hosts and animal caches as food sources. Microbial and/or fungal colonization of grain or wood may have been historically helpful for the successful feeding and oviposition by R. dominica, because it weakens the grain kernel’s bran (e.g., outer shell) in the case of stored products or weakens the bark in the case of woody natural hosts, allowing individuals of a variety of taxa easier access (Biedermann et al. 2019). Rhyzopertha dominica is a bostrichid, a family of wood-boring beetles that also includes another important stored product pest, Prostephanus truncates Horn, which has a documented affinity for stressed or already girdled trees in natural landscapes (Quellhorst et al. 2021). Indeed, R. dominica is readily captured far from food facilities among native habitats (Jia et al. 2008; Mahroof et al. 2010), suggesting that host switching may occur. While foraging, R. dominica may use MVOC’s in enclosed environments to navigate, their use of these cues in the field should be confirmed. Thus, our study helps to highlight the importance of microbial cues for foraging of some stored product insects, depending on their life history. In addition, our results suggest that future work elucidating the interaction of R. dominica with the microbial community may be worthwhile.
Unlike R. dominica, we did not find elevated attraction to MVOC’s by the secondary pest, T. castaneum. As a secondary pest, T. castaneum is not able to access resources in intact grain (Hagstrum and Subramanyam 2006). As a consequence, it is especially surprising that T. castaneum did not respond to the MVOC’s in this study, because colonization by a low number of microbes may be able to weaken a kernel sufficiently so that it could be used by T. castaneum. Indeed, prior work has documented that, after damage by a primary pest, wheat kernels were more attractive to T. castaneum than intact or mechanically-damaged kernels (Trematerra et al. 2000), suggesting that invasion of a grain mass by species like R. dominica and Sitophilus oryzae (L.) (Coleoptera: Curculionidae) may facilitate colonization of T. castaneum. Thus grain conditioned or infested with Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) was in fact found to be preferred by T. castaneum in olfactometer assays (Trematerra and Ianiro 2015). It is clear that T. castaneum then may follow primary pests to access nutrients in intact grain more easily, but this makes the lack of response to cues from another pest that could open up an otherwise inaccessible resource even more unexpected. The varying response by species with different life histories to MVOC’s further highlights the importance of understanding the ecological interactions between insects and fungi in the postharvest environment.
The lack of response by T. castaneum may partly be explained by the setup of the current study. The MVOC’s in this study originated from any fungi that were already present on the grain at the beginning of the experiment. In fact, we confirmed that there was a diverse community of fungi that had colonized the grain, with Alternaria sp. and Aspergillus sp. as the most common groups present. In total, we isolated nine species of fungi. Multiple species of microbes in the grain may have been emitting behaviorally antagonistic volatiles that could have produced no net change in attraction. Varying blends of volatiles are produced by specific fungal species that colonize grain (Magan and Evans 2000). This has implications for attraction and preference by the stored product insect community. For example, Tenebrio molitor L. (Coleoptera: Tenebrionidae) avoided grain infested with Fusarium avanaceum or Beauveria bassiana, but were attracted to grain with F. proliferatum, F. poae, or F. culmorum (Guo et al. 2014). Ahmad et al. (2012) found moldy cotton lint to be attractive to T. castaneum; in follow-up tests, the authors cultured five unidentified fungal strains isolated from the cotton and found conspecifics were attracted to one of the strains, repulsed by another, whereas the other three had no behavioral effects. Thus, depending on the relative abundance of microbial species in the grain, and the strength of behavioral response by the insect community, the signal-to-noise ratio may be too high to detect a behavioral change. If true, this also suggests that R. dominica is potentially broadly attracted to a greater range of MVOC’s than T. castaneum. While Rering et al. (2020) found that response to microbial monocultures and microbial assemblages were similar by the European honey bee, Apis mellifera, the generalization of this finding is currently unknown, and may vary in other systems. Future research should evaluate behavioral response to microbial monocultures found from grain in the postharvest environment to tease apart which species might be producing volatiles of behavioral relevance to stored product insects.
In this study, we tempered grain with free water, a common milling technique, to elevate grain moisture between 12% and 19%, which roughly conforms to the practical range of tempering used in the wheat milling industry (12–17%) to facilitate separation of the bran and germ from white flour (Carter et al. 2015). While the incubation periods in this study were longer than those typically found at food facilities, it did offer microbes a chance for proliferation, and microbial growth was confirmed as a source of volatiles over grain by the novice sensory panel in this study. Another approach to allowing microbial growth in the grain would have been conditioning grain in salt chambers to equilibrate to atmospheric moisture conditions over time, but this would have required significantly longer periods of time; time could not have been varied independently. Prior work using this methodology found the primary fungal species on maize after 348 and 751 d consisted of 20 species, including Acremonium zeae, A. flavus, Penicillium pinophilum, and others (Wicklow et al. 1998).
Prior literature has described a variety of aldehydes, alcohols, ketones, alkanes, and alkenes that are characteristic of wheat (Mattiolo et al. 2017). Common fungal volatiles include 1-octen-3-ol, 3-octanol, 3-methyl-butanol, and 3-octanol (Herrera et al. 2015; Morath et al. 2012; Ponce et al. 2021), and some of these were in the various tempered wheat treatments. For example, in the 19% moisture treatment incubated for 18 d or 27 d, 1-octen-3-ol was a significant constituent (Supplementary Table 1). The fact that certain fungal metabolites were found in some tempered treatments, but not all, suggests that the fungal community may not have been identical among tempered treatments. Ponce et al. (2021) recently found that the behavioral response by the stored product insect community to MVOC’s may be context and species dependent. Knowing which of the volatiles in these complex headspaces are perceived by stored product insects may be done in the future by gas chromatography coupled with electroantennography to help develop a list of target compounds for future work. This will help prioritize which specific compounds to test for behavioral experiments in order to develop behaviorally relevant semiochemicals to support postharvest protection of commodities.
Finally, interactions between the insect and microbial communities are important in the postharvest environment, and may have important implications for grain quality (Quellhorst et al. 2021). Even when there is no visible mold growth, there may imperceptible (e.g., to human eyes) but consistent changes in the grain in the near infrared, confirmed by sequencing microbes on the grain, and emission of off-odors that are deleterious to grain quality, as in this study. Changes in volatile profiles have been used as indicators of food and feed spoilage (Schnürer et al. 1999). Moreover, prior work has found that insects and microbes may alter grain environments in ways that are mutually beneficial (Ponce et al. 2021), for example by altering the microclimate in grain masses in ways that enhance the fitness of the interacting partner. Often, the management of insects and microbes is considered separately at food facilities, but our study highlights that they may significantly affect each other and may need to be managed simultaneously.
Data Availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Van Winkle, Taylor; Ponce, Marco; Quellhorst, Hannah E.; Bruce, Alexander; Albin, Chloe, E.; Kim, Tania N.; Zhu, Kun Yan; Morrison, William R.. Dataset for “Microbial volatile organic compounds mediate attraction by a primary but not secondary stored product insect pest in wheat”. Ag Data Commons. https://data.nal.usda.gov/dataset/dataset-microbial-volatile-organic-compounds-mediate-attraction-primary-not-secondary-stored-product-insect-pest-wheat. Accessed 2021-02-19.
Code Availability
Code is available upon request to corresponding author.
References
Adams RP (2009) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Co., Carol Stream, IL
Ahmad F, Daglish GJ, Ridley AW et al (2013) Short-range resource location by Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) demonstrates a strong preference for fungi associated with cotton seed. J Stored Prod Res 52:21–27. https://doi.org/10.1016/j.jspr.2012.09.003
Ahmad F, Daglish GJ, Ridley AW, Walter GH (2012) Responses of Tribolium castaneum to olfactory cues from cotton seeds, the fungi associated with cotton seeds, and cereals. Entomol Exp Appl 145:272–281. https://doi.org/10.1111/eea.12012
Altschul SF, Madden TL, Schäffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1080/14620316.2005.11511891
Bachy A, Aubinet M, Amelynck C, Schoon N, Bodson B, Delaplace P, De Ligne A, Digrado A, du Jardin P, Fauconnier M-L, Mozaffara A, Müller J-F, Heinesch B (2020) Dynamics and mechanisms of volatile organic compound exchanges in a winter wheat field. Atmos Environ 221:117105
Bashir T, Birkinshaw LA, Hall DR, Hodges RJ (2001) Host odours enhance the responses of adult Rhyzopertha dominica to male-produced aggregation pheromone. Entomol Exp Appl 101:273–280. https://doi.org/10.1023/A:1019282901748
Beck JJ, Vannette RL (2017) Harnessing insect-microbe chemical communications to control insect pests of agricultural systems. J Agric Food Chem 65:23–28. https://doi.org/10.1021/acs.jafc.6b04298
Beck JJ, Alborn HT, Block AK, Christensen SA, Hunter CT, Rering CC, Seidl-Adams I, Stuhl CJ, Torto B, Tumlinson JH (2018) Interactions among plants, insects, and microbes: elucidation of inter-organismal chemical communications in agricultural ecology. J Agric Food Chem 66:6663–6674
Belkacem-Hanfi N, Semmar N, Perraud-Gaime I, Guesmi A, Cherni M, Cherif I, Boudabous A, Roussos S (2013) Spatio-temporal analysis of post-harvest moulds genera distribution on stored durum wheat cultivated in Tunisia. J Stored Prod Res 55:116–123
Biedermann PHW, Müller J, Gregoire J-C et al (2019) Bark beetle population dynamics in the Anthropocene: Challenges and solutions. Trends Ecol Evol 34:914–924
Bueno E, Martin KR, Raguso RA et al (2020) Response of wild spotted wing drosophila (Drosophila suzukii) to microbial volatiles. J Chem Ecol 46:688–698. https://doi.org/10.1007/s10886-019-01139-4
Buhl J, Rogers S (2016) Mechanisms underpinning aggregation and collective movement by insect groups. Curr Opin Insect Sci 15:125–130. https://doi.org/10.1016/j.cois.2016.04.011
Campbell JF (2012) Attraction of walking Tribolium castaneum adults to traps. J Stored Prod Res 51:11–22. https://doi.org/10.1016/j.jspr.2012.06.002
Carter BP, Galloway MT, Morris CF, Weaver GL, Carter AH (2015) The case for water activity as a specification for wheat tempering and flour production. Cereal Foods World 60:166–170
Cordeiro EMG, Campbell JF, Phillips T (2019) Differences in orientation behavior and female attraction by Rhyzopertha dominica (Coleoptera: Bostrichidae) in a homogeneous resource patch. Environ Entomol 48:784–791. https://doi.org/10.1093/ee/nvz058
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859. https://doi.org/10.1007/s10886-013-0306-z
Davis TS, Landolt PJ (2013) A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J Chem Ecol 39:860–868. https://doi.org/10.1007/s10886-013-0278-z
Dissanayaka DMSK, Sammani AMP, Wijayaratne LKW, et al (2020) Effects of aggregation pheromone concentration and distance on the trapping of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) adults. J Stored Prod Res 88:101657. https://doi.org/10.1016/j.jspr.2020.101657
Dunkel FV (1988) The relationship of insects to the deterioration of stored grain by fungi. Intl J Food Microbiol 7:227–244
Edde PA, Phillips TW, Toews MD (2005) Responses of Rhyzopertha dominica (Coleoptera: Bostrichidae) to its aggregation pheromones as influenced by trap design, trap height, and habitat. Environ Entomol 34:1549–1557. https://doi.org/10.1603/0046-225X-34.6.1549
Edde PA, Phillips TW (2006) Potential host affinities for the lesser grain borer, Rhyzopertha dominica: Behavioral responses to host odors and pheromones and reproductive ability on non-grain hosts. Entomol Exp Appl 119:255–263. https://doi.org/10.1111/j.1570-7458.2006.00417.x
Edde PA, Toews MD, Phillips TW (2011) Effects of various semiochemicals on the responses of Rhyzopertha dominica to pheromone traps in the field. Ann Entomol Soc Am 104:1297–1302. https://doi.org/10.1603/AN11090
Green PWC (2008) Fungal isolates involved in biodeterioration of book-paper and their effects on substrate selection by Liposcelis bostrychophila (Badonnel) (Psocoptera: Liposcelididae). J Stored Prod Res 44:258–263. https://doi.org/10.1016/j.jspr.2008.01.003
Guo Z, Dol̈l K, Dastjerdi R, et al (2014) Effect of fungal colonization of wheat grains with Fusarium spp. on food choice, weight gain and mortality of meal beetle larvae (Tenebrio molitor). PLoS One 9:e100112. https://doi.org/10.1371/journal.pone.0100112
Hagstrum DW, Phillips TW (2017) Evolution of stored-product entomology: protecting the world food supply. Annu Rev Entomol 62:379–397. https://doi.org/10.1146/annurev-ento-031616-035146
Hagstrum DW, Subramanyam B (2006) Fundamentals of Stored-Product Entomology. AACC International, St. Paul
Herrera JM, Pizzolitto RP, Zunino MP et al (2015) Effect of fungal volatile organic compounds on a fungus and an insect that damages stored maize. J Stored Prod Res 62:74–80. https://doi.org/10.1016/j.jspr.2015.04.006
Hertz M, Jensen IR, Jensen LØ, Thomsen SN, Winde J, Dueholm MS, Sørensen LH, Wollenberg RD, Sørensen HO, Sondergaard TE, Sørensen JL (2016) The fungal community changes over time in developing wheat heads. Intl J Food Microbiol 222:30–39
Hoftstetter RW, Dempsey TD, Klepzig KD, Ayres MP (2007) Temperature-dependent effects on mutualistic, antagonistic, and commensalistic interactions among insects, fungi and mites. Community Ecol 8:47–56. https://doi.org/10.1556/ComEc.8.2007.1.7
Hothorn T (2017) Additional multcomp Examples. 1–11
Jia F, Toews MD, Campbell JF, Ramaswamy SB (2008) Survival and reproduction of lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) on flora associated with native habitats in Kansas. J Stored Prod Res 44:366–372
Kamil AC, Gould KL (2008) Memory in food caching animals. Learning and memory: a comprehensive reference. Elsevier, Amsterdam, pp 419–439
Leos-Martinez J, Granovsky TA, Williams HJ et al (1987) Pheromonal trapping methods for lesser grain borer, Rhyzopertha dominica (Coleoptera: Bostrichidae). Environ Entomol 16:747–751. https://doi.org/10.1093/ee/16.3.747
Liu W, Longnecker M, Tarone AM, Tomberlin JK (2016) Responses of Lucilia sericata (Diptera: Calliphoridae) to compounds from microbial decomposition of larval resources. Anim Behav 115:217–225. https://doi.org/10.1016/j.anbehav.2016.03.022
Magan N, Evans P (2000) Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J Stored Prod Res 36:319–340. https://doi.org/10.1016/S0022-474X(99)00057-0
Mahroof RM, Edde PA, Robertson B et al (2010) Dispersal of Rhyzopertha dominica (Coleoptera: Bostrichidae) in different habitats. Environ Entomol 39:930–938. https://doi.org/10.1603/EN09243
Mattiolo E, Licciardello F, Lombardo GM et al (2017) Volatile profiling of durum wheat kernels by HS–SPME/GC–MS. Eur Food Res Technol 243:147–155. https://doi.org/10.1007/s00217-016-2731-z
Morath SU, Hung R, Bennett JW (2012) Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol Rev 26:73–83. https://doi.org/10.1016/j.fbr.2012.07.001
Morrison WR, Allen M, Leskey TC (2018a) Behavioural response of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to host plant stimuli augmented with semiochemicals in the field. Agric for Entomol 20:62–72. https://doi.org/10.1111/afe.12229
Morrison WR, Blaauw BR, Short BD et al (2019a) Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag Sci. https://doi.org/10.1002/ps.5156
Morrison WR, Bruce A, Wilkins RV, Albin CE, Arthur FH (2019b) Sanitation improves stored product insect pest management. Insects 10:77
Morrison WR, Scully ED, Campbell JF (2021) Towards developing semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag Sci 77:2667–2682
Morrison WR, Wilkins RV, Gerken AR et al (2018b) Mobility of adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after exposure to long-lasting insecticide-incorporated netting. J Econ Entomol 111:2443–2453. https://doi.org/10.1093/jee/toy173
Murdock LL, Margam V, Baoua I, Balfe S, Shade RE (2012) Death by desiccation: effects of hermetic storage on cowpea bruchids. J Stored Prod Res 49:166–170
Nguyen DT, Hodges RJ, Belmain SR (2008) Do walking Rhyzopertha dominica (F.) locate cereal hosts by chance? J Stored Prod Res 44:90–99. https://doi.org/10.1016/j.jspr.2007.06.008
Oi CA, van Zweden JS, Oliveira RC et al (2015) The origin and evolution of social insect queen pheromones: Novel hypotheses and outstanding problems. BioEssays 37:808–821. https://doi.org/10.1002/bies.201400180
Oksanen J, Blanchet FG, Friendly M, et al (2019) vegan. 1–296
Pearson T, Maghirang E, Dowell F (2013) A multispectral sorting device for wheat kernels. Am J Agric Sci Technol 2:45–60. https://doi.org/10.7726/ajast.2013.1004
Perišic V, Perišic V, Vukajlovic F, Pešic S, Predojevic DV, Lukovic K (2017) Feeding preferences and progeny production of Rhyzopertha dominica (Fabricius 1792) (Coleoptera: Bostrichidae) in small grains. Biologica Nyssana 9:55–61
Pickett JA, Woodcock CM, Midega CAO, Khan ZR (2014) Push-pull farming systems. Curr Opin Biotechnol 26:125–132. https://doi.org/10.1016/j.copbio.2013.12.006
Pierce AM, Borden JH, Oehlschlager AC (1983) Effects of age and population density on response to beetle and food volatiles by Oryzaephilus surinamensis and O. mercator (Coleoptera: Cucujidae). Environ Entomol 12:1367–1374. https://doi.org/10.1093/ee/12.5.1367
Pierce AM, Pierce HD, Borden JH, Oehlschlager AC (1991) Fungal volatiles: semiochemicals for stored-product beetles (Coleoptera: Cucujidae). J Chem Ecol 17:581–597
Ponce M, Kim TN, Morrison WR (2021) The behavioral response by stored product insects to microbial volatiles. Insects 12:391
Quellhorst H, Athanassiou CG, Zhu KY, Morrison IWR (2021) The biology, ecology, and management of the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). J Stored Prod Res, in press.
Rering CC, Vannette RL, Schaeffer RN, Beck JJ (2020) Microbial co-occurrence in floral nectar affects metabolites and attractiveness to a generalist pollinator. J Chem Ecol 46:659–667. https://doi.org/10.1007/s10886-020-01169-3
Ridley AW, Hereward JP, Daglish GJ et al (2011) The spatiotemporal dynamics of Tribolium castaneum (Herbst): Adult flight and gene flow. Mol Ecol 20:1635–1646. https://doi.org/10.1111/j.1365-294X.2011.05049.x
Rork AM, Renner T (2018) Carabidae semiochemistry: Current and future directions. J Chem Ecol 44:1069–1083. https://doi.org/10.1007/s10886-018-1011-8
Russell AL, Ashman TL (2019) Associative learning of flowers by generalist bumble bees can be mediated by microbes on the petals. Behav Ecol 30:746–755. https://doi.org/10.1093/beheco/arz011
R Core Team (2021) R: A language and environment for statistical computing. https://www.r-project.org/
Schnürer J, Olsson J, Börjesson T (1999) Fungal volatiles as indicators of food and feeds spoilage. Fungal Genetics Biol 27:209–217
Trematerra P, Ianiro R (2015) Behavioral interactions between Sitophilus zeamais and Tribolium castaneum: the first colonizer matters. J Pest Sci 88:573–581. https://doi.org/10.1007/s10340-014-0633-z
Trematerra P, Sciarreta A, Tamasi E (2000) Behavioural responses of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum to naturally and artificially damaged durum wheat kernels. Entomol Exp Appl 94:195–200. https://doi.org/10.1023/A:1003929810978
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Weisskopf L, Schulz S, Garbeva P (2021) Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat Rev Microbiol. https://doi.org/10.1038/s41579-020-00508-1
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Wicklow DT, Weaver DK, Throne JE (1998) Fungal colonists of maize grain conditioned at constant temperatures and humidities. J Stored Prod Res 34:355–361
Wilkins RV, Campbell JF, Zhu KY, et al (2021) Long-lasting insecticide-incorporated netting and interception traps at pilot-scale warehouses and commercial facilities prevents infestation by stored product beetles. Front Food Syst 4:561820.
Xu H, Turlings TCJ (2018) Plant volatiles as mate-finding cues for insects. Trends Plant Sci 23:100–111. https://doi.org/10.1016/j.tplants.2017.11.004
Ziegler V, Paraginski RT, Ferreira CD (2021) Grain storage systems and effects of moisture, temperature and time on grain quality - a review. J Stored Prod Res 91: 101770.
Zunino MP, Herrera JM, Pizzolitto RP et al (2015) Effect of selected volatiles on two stored pests: The fungus Fusarium verticillioides and the maize weevil Sitophilus zeamais. J Agric Food Chem 63:7743–7749. https://doi.org/10.1021/acs.jafc.5b02315
Acknowledgements
We would like to thank the excellent technical assistance of the whole Morrison Lab (USDA). In addition, we acknowledge the substantive feedback of Dr. Ann Fraser (Kalamazoo College), as well as Meghan Horal, Grant Lobert, Rose Maylen, MP, and Elias Tolbert, which constituted the institutional advisor and peer review group for an earlier draft of this senior individualized project at Kalamazoo College in the Biology Department. The use of trade names is for the purposes of providing scientific information only, and does not constitute endorsement by the United States Department of Agriculture and Kansas State University (KSU). The USDA and KSU are equal opportunity employers. This manuscript is contribution No. 21-216-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, KS, USA.
Funding
This work was funded, in part, by a United States Department of Agriculture, National Institute of Food and Agriculture, Crop Protection and Pest Management Grant No. 2017-70006-27262. Further, this work was funded by an ARS Innovation Funds Account#992-0142-901 Sub 3 and USDA-APHIS PPQ Agreement#60-3020-1-003 (108-3020-001).
Author information
Authors and Affiliations
Contributions
TVW and WRM conceived of the experiments. TVW, MP, RVW, and CEA collected the data. WRM and AB analyzed the data. TVW developed an initial draft with input from WRM. All authors edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethical Approval
The authors have adhered to all applicable ethics guidelines in the US and at each institution. This work was reviewed and approved by the Kansas State University Institutional Biosafety Committee and covered under IBC Registration#1437 managed by WRM.
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved of authorship on this publication.
Conflict of Interest
The authors declare that they have no conflict of interests with the current work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Winkle, T., Ponce, M., Quellhorst, H. et al. Microbial Volatile Organic Compounds from Tempered and Incubated Grain Mediate Attraction by a Primary but Not Secondary Stored Product Insect Pest in Wheat. J Chem Ecol 48, 27–40 (2022). https://doi.org/10.1007/s10886-021-01312-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01312-8