Abstract
Transcutaneous PCO2 (PTCCO2) and end-tidal PCO2 (PETCO2) measurement methods serve as alternatives to arterial PCO2 (PaCO2), providing continuous non-invasive monitoring. The objective of this study was to evaluate the PTCCO2 and PETCO2 methods with actual PaCO2 levels, and to assess the variability of measurements in relation to subject-related factors, such as skin and subcutaneous adipose tissue thickness and presence of pulmonary diseases. PTCCO2, PETCO2 and PaCO2 were measured at the same time in intubated pediatric subjects. Subjects’ demographic characteristics, clinical features, laboratory parameters, skin and subcutaneous adipose tissue thickness were identified. The study was carried out on 102 subjects with a total of 1118 values for each method. In patients with non-pulmonary disease, the mean difference between PTCCO2 and PaCO2 was − 0.29 mmHg (± 6.05), while it was 0.44 mmHg (± 6.83) bias between PETCO2 and PaCO2. In those with pulmonary diseases, the mean difference between PTCCO2 and PaCO2 was − 1.27 mmHg (± 8.32), while it was − 4.65 mmHg (± 9.01) between PETCO2 and PaCO2. Multiple linear regression demonstrated that increased subcutaneous adipose tissue thickness, core body temperature and inotropic index were related with higher PTCCO2 values relative to the actual PCO2 values. Other factors, such as skin tissue thickness, presence of pulmonary disease, measurement location and measurement times were non-significant. The PTCCO2 method has higher reliability than the PETCO2 method, and PTCCO2 measurements are not influenced by most subject-related factors; however, core body temperature, inotropic index and subcutaneous adipose tissue thickness can lead to significant differences in PCO2 measurement.
Similar content being viewed by others
1 Introduction
The monitoring of carbon dioxide (CO2) level is essential for diagnosis and therapeutic guidance in mechanically ventilated and/or tracheostomized subjects [1]. Subjects with parenchymal or non-parenchymal lung disease with invasive ventilation must be monitored to assess alveolar ventilation and also to predict the need for mechanical ventilation (MV) [1]. The current gold standard method for the measurement of partial pressure of carbon dioxide (PCO2) is intermittent arterial blood gas (ABG) analysis. In addition to being a time-consuming invasive method, ABG does not provide continuous monitoring and measures arterial PCO2 (PaCO2) with arterial puncture which may be associated with increased blood loss, potentially-permanent vessel damage and catheter associated complications. Also it does not provide real-time measurement of PCO2; delaying response time in critically ill patients [2]. However, although CO2 monitoring has several non-invasive measurement techniques, PaCO2 analysis remains as the gold standard method. With today's technology, it is not possible for any non-invasive method to entirely replace PaCO2 measurements.
Ideally, non-invasive techniques of measurement should be available for continuous monitoring of oxygenation and ventilation status. For instance, pulse oximetry has proven to be a rapid, reliable and non-invasive measurement of oxygen saturation by using a non-adhesive skin sensor, but there is no similar method for measuring CO2 levels transcutaneously [3]. Transcutaneous PCO2 (PTCCO2) and end-tidal PCO2 (PETCO2) measurements serve as alternatives to PaCO2 measurement and provide continuous and non-invasive monitoring of subject. The essence of non-invasive gas monitoring is to provide information about alveolar ventilation and circulatory gas levels without the need for repetitive blood sampling [1,2,3,4].
PETCO2 monitoring via capnometer provides information on the adequacy of ventilation and displays the waveform of PCO2 in exhaled air [4]. Detection of exhaled PCO2 has proven to be a valuable mechanism to confirm tracheal intubation and recognize accidental esophageal intubations, among other critical patient safety benefits [2]. The safety enhancements provided by CO2 monitoring also include the detection of invasive airway disconnection, dislodgement or obstruction, postoperative monitoring of respiratory depression, prediction of underlying airway or lung pathologies, and monitoring the effectiveness of cardiopulmonary resuscitation [5, 6].
PTCCO2 monitors perform measurements based on the capillary bed and provide continuous information about transcutaneous CO2 through the local application of heat and measurement by electrodes [7]. Transcutaneous monitors have been more widely used in neonates because of their thinner skin which minimizes resistance to gas diffusion [8]. There are numerous studies which show good correlations between non-invasive carbon dioxide measurement methods and PaCO2 values, both in the pediatric [9,10,11,12,13] and adult population [14, 15]. However, other authors have not been able to confirm these results, while some studies demonstrate conflicting findings [16,17,18].
The objective of this study was to evaluate the relationships between the PTCCO2 and PETCO2 methods and the gold standard ABG analysis in mechanically ventilated children in the pediatric intensive care unit. The secondary objective was to assess the variability of PTCCO2 measurements in relation to subject-related factors, such as skin and subcutaneous adipose tissue thickness and pulmonary diseases.
2 Materials and methods
2.1 Subjects
This is a single-center, prospective and comparative study approved by the Clinical Research Ethics Committee of Istanbul Medeniyet University Goztepe Training and Research Hospital (study registration number: 2017-9375).
The study evaluated all children aged between 1 month and 17 years that had been intubated with cuffed ETT due to a definite indication for mechanical ventilation. The intubations were performed with single-lumen cuffed ETT with appropriate size for age and weight. Among these patients, those who accepted invasive monitoring of arterial blood pressure and provided informed consent (from the parents or legal guardians) were included in the study. The presence of any one of the following characteristics was defined as grounds for exclusion from the study: sampling performed with venous blood, non-compliance to the study protocol (premature discontinuation of measurement, incorrect installation of sensor or signal abnormality of monitor or backup), use of uncuffed endotracheal tubes, determination of any type of air leakage in the lung (pneumothorax, pneumomediastinum etc.).
2.2 Measurements
We used two non-invasive CO2 measurement methods (end-tidal CO2; PetCO2 and transcutaneous CO2; PtcCO2) and an invasive CO2 measurement method (PaCO2) via ABG, in mechanically ventilated children admitted to Istanbul Medeniyet University Hospital, Pediatric Intensive Care Unit (PICU) between November 2017 and June 2019.
2.2.1 Transcutaneous CO2 measurements
Transcutaneous CO2 was measured by using a TCM4 PTCCO2/PTCO2 device (Radiometer™, Copenhangen, Denmark, TCM4® series CombiM). The electrode membrane device was cleaned and calibrated at the beginning of measurement and repetitive calibration was applied every four hours. A small drop of sensor gel was applied to the center of the sensor membrane’s surface. The purpose of using sensor gel is to enable gas diffusion by moisturizing the skin. The electrode temperature was set to 44.0 °C to enhance sufficient blood flow in the capillaries to allow for PCO2 measurement in accordance with the manufacturer’s recommendations. There are three preselected locations in the supine position: (a) second intercostal space in the midclavicular line, (b) lateral surface of abdomen at the level of the umbilicus in the midclavicular line and (c) inner surface of the thigh. New fixation rings were used at each transcutaneous CO2 measurement location. The transcutaneous sensor was applied to the child’s chest, thigh or abdomen and was allowed to stabilize for at least 15 min prior to data recording.
2.2.2 End-tidal CO2 measurements
The CO2 sensor (Mainstream Capnostat 5 EtCO2 Sensor, Philips Healthcare, Eindhoven, Netherlands) was placed next to the tracheal cannula or intubation tube and was connected to the monitor (MX 600 Philips Intellivue™, Amsterdam, The Netherlands) for display. Calibration of the PETCO2 sensor was performed by zeroing of the sensor in room air. Calibration was done prior to measurements for each subject.
2.2.3 Arteriel blood gas analysis
PCO2 measurements from ABG were analyzed at the bedside using an ABL 90 FLEX blood gas analyzer (Radiometer, Medical ApS, Copenhagen, Denmark) within 3 min of collection. As soon as blood samples were taken for ABG analysis, PTCCO2 and PETCO2 measurements were recorded simultaneously.
2.2.4 Measurement of skin and subcutaneous adipose tissue thickness
The same radiologist performed skin and subcutaneous adipose tissue thickness measurements via ultrasonography at the point where transcutaneous CO2 sensors were placed. A linear L12-3 probe was used (EPIQ 7C, Philips, Bothell, Seattle, WA, USA). Patients were in the supine position and measurements were performed without applying pressure to the probe at the CO2 ring localizations (chest, abdomen and thigh).
2.3 Study procedure
Transcutaneous CO2 measurement was initiated from chest location in each subject. Then, thigh and abdomen measurements were taken respectively. At the 15th minute and 3rd hour after sensor fixation and calibration, PTCCO2–PETCO2 and PaCO2 measurements were recorded simultaneously for each location starting from the chest location (Fig. 1). The measurement protocol was planned to be performed in two cycles per subject –each cycle containing 6 readings (chest, thigh and abdomen readings on the 15th minute and 3rd hour), unless subjects expired or were extubated before the two cycles were complete. Subjects who could not complete at least one cycle protocol (at least two measurements per location with a total of 6 readings) were excluded from all analyses (Fig. 2). The results were recorded after sensor fixation at three locations sequentially and were compared with PaCO2 and PETCO2 results that were measured simultaneously.
Finally, a total of 1118 pairs of measurements were recorded for each measurement method. The maximum acceptable difference between PaCO2 and non-invasive CO2 measurements (PTCCO2 and PETCO2) was defined as ± 4 mmHg [19].
The following demographic characteristics, clinical features and laboratory parameters of subjects were identified: sex, age (month) and core body temperature (sensor in the esophagus). Parameters of mechanical ventilation were also recorded, including FiO2, peak pressure (Ppeak) and mean airway pressure (MAP). Measurement of the non-invasive CO2 values (PTCCO2 and PETCO2), parameters of ABG analysis (pH, PaCO2, PaO2, HCO3ˉ, base excess, haemoglobin and lactate level), inotropic index (inotropic index = dose of dopamine + dobutamine + [100 × epinephrine] + [100 × norepinephrine] + [15 × milrinone] [in microgram/kg/min]) and oxygenation index (OI) (OI = [FiO2 × MAP × 100)/PaO2]) [20, 21] were also included among the parameters of the study.
For subgroup analysis, subjects were divided into two groups according to presence of pulmonary disease. In these two groups, subjects with pulmonary disease (PD) were defined as MAP ≥ 14 mmHg and/or OI ≥ 10, and subjects with non-pulmonary disease (Non-PD) were defined as MAP < 14 mmHg and/or OI < 10). PaCO2 values were compared with PTCCO2 and PETCO2 values in both groups.
Finally, we also determined the variability in transcutaneous CO2 measurement results and various parameters, including skin and subcutaneous adipose tissue thickness, presence of PD, measurement time, and measurement location.
2.4 Statistical analysis
Analyses were performed by using the SPSS version 21 (SPSS, Inc., Chicago, IL) or Med Calc v15.8 (Med Calc Software, Ostend, Belgium) software. Subject characteristics are described using qualitative variables (using frequencies and percentages) and quantitative variables (using means and standard deviation [SD] or median with interquartile range [IQR] depending on type of distribution). Simple linear regression analysis was performed and Pearson correlation coefficients were calculated for the assessment of the relationships between PaCO2, PTCCO2 and PETCO2. CO2 values of the different methods were compared by using Friedman’s test with Bonferroni correction method for all measurements and the Wilcoxon Signed Rank test for specific locations of PTCCO2 values. Bland–Altman plots were created to evaluate the agreement between measurements. We also performed multiple linear regression analysis with stepwise selection method to determine factors affecting PTCCO2 values. Variables with a p-value lower or equal to 0.10 in univariate analysis were included into the model. P < 0.05 values were accepted as statistically significant.
3 Results
The study was performed in 102 subjects with 1118 measurements for each method. The descriptive factors of the study are shown in Table 1. The tolerance of skin to the electrode was quite good; there were no signs of skin irritation or erythema at the end of monitoring. The trial flow chart is shown in Fig. 2.
3.1 Comparison of the two non-invasive PCO2 methods with ABG analysis results
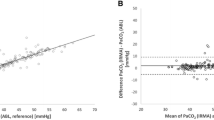
The median PaCO2, PTCCO2 and PETCO2 values were 38.9 (IQR: 34.2–44.4), 38 (IQR: 34–43) and 37 (IQR: 32–44) mmHg, respectively. Results of the Bland–Altman analysis comparing PTCCO2/PaCO2 and PETCO2/PaCO2 pairs are summarized in Table 2 and illustrated in Fig. 3 with regard to all subject groups and also subgroups. In all subjects, the mean difference between PTCCO2 and PaCO2 was − 0.78 (± 7.29) (95% limits of agreement − 15.06 to 13.51 mmHg) with moderate correlation (r = 0.66, p < 0.001) (Fig. 3a). Similarly, the mean bias between PETCO2 and PaCO2 was -2.10 (± 8.39) (95% -18.54 to 14.33 mmHg) with moderate correlation (r = 0.51, p < 0.001) (Fig. 3b). Although both PTCCO2 and PETCO2 were moderately correlated, the correlation coefficient of PTCCO2 was higher.
Bland–Altman plots for mean PTCCO2 versus PaCO2 and mean PETCO2 versus PaCO2. PaCO2 and PTCCO2 for all subjects (a), PaCO2 and PETCO2 for all subjects (b), PaCO2 and PTCCO2 for the subjects with non-pulmonary disease (c), PaCO2 and PETCO2 for the subjects with non-pulmonary disease (d), PaCO2 and PTCCO2 for the subjects with pulmonary disease (e), PaCO2 and PETCO2 for the subjects with pulmonary disease (f). The mean difference is represented as a continuous line, and 95% limits of agreement are represented as dotted lines
According to our findings, reliable PCO2 measurements (within the predefined, clinically acceptable range of ± 4 mmHg) could be achieved by the PTCCO2 method, but not by the PETCO2 method. The difference between PaCO2 and PTCCO2 was ≤ ± 4 mmHg in 662 measurements out of 1118 (59.2%) while the difference between the PaCO2 and PETCO2 was ≤ ± 4 mmHg in 471 measurements (42.1%) (p = 0.001).
In Fig. 4, PTCCO2 and PETCO2 measurements are illustrated for all subjects. It was found that a 1 mm Hg increase in PTCCO2 values was associated with a 0.55 mm Hg increase in PETCO2 values.
3.2 Subgroup analyses and comparisons
Among the subjects, 46.1% (n = 47) had PD and 53.9% (n = 55) of subjects were without pulmonary disease (non-PD). In the non-PD group, the mean bias between PTCCO2 and PaCO2 was -0.29 (± 6.05) (95% limits of agreement − 12.15 to 11.57 mmHg) (Fig. 3c), while the mean bias between PETCO2 and PaCO2 was 0.44 (± 6.83) (95% limits of agreement − 12.95 to 13.83 mmHg) (Fig. 3d). Correlation coefficients were r = 0.67 (p < 0.001) and r = 0.52 (p < 0.001), respectively. In the PD group, the mean bias between PTCCO2 and PaCO2 was − 1.27 (± 8.32) (95% limits of agreement − 17.57 to 15.04 mmHg) (Fig. 3e). Whereas the mean bias between PETCO2 and PaCO2 was − 4.65 (± 9.01) (95% limits of agreement − 22.30 to 13.01 mmHg) (Fig. 3f). Although the mean bias for PTCCO2 and PETCO2 were increased in the presence of PD, PTCCO2 was better correlated with PaCO2, compared to PETCO2 (respectively: r = 0.61, p < 0.001 vs. r = 0.53, p < 0.001).
We found that the absolute values of PTCCO2–PaCO2 were significantly lower than the absolute values of PETCO2–PaCO2 for all subjects (p < 0.001), the non-PD group (p < 0.001) and also the PD group (p < 0.001) (Table 2).
3.3 The variability in PTCCO2 measurements in relation to subject-related factors
We performed multiple linear regression analysis with PTCCO2–PaCO2 as a dependent variable to determine factors affecting differences between the measurements. We found that increased subcutaneous adipose tissue thickness (p = 0.007), body temperature (p < 0.001) and inotropic index (p = 0.002) were related with higher PTCCO2 values relative to actual PaCO2 values (Table 3). The other factors included in the model, such as age (p = 0.061), gender (p = 0.151), skin tissue thickness (p = 0.571), PaO2 (p = 0.725), presence of PD (p = 0.134), measurement time (p = 0.299), and measurement location (p = 0.121) were found to be non-significant.
4 Discussion
To our knowledge, this is the most comprehensive comparison between two non-invasive techniques for continuous measurement of CO2 in pediatric subjects undergoing invasive mechanical ventilation in the PICU. It is also the largest cohort study of PTCCO2 and PETCO2 measurement in mechanically ventilated subjects with 1118 measurements for each method. We also compared PTCCO2 values with subjects’ characteristics to determine their effects on methods of PaCO2 measurement. Our results demonstrated the superiority of PTCCO2 monitoring over PETCO2 in mechanically-ventilated critically ill subjects, as demonstrated by the differences between PaCO2 values and the two methods’ results (PTCCO2 and PETCO2).
In all subject groups, the mean bias between PTCCO2 and PaCO2 was − 0.78 mmHg (± 7.29) (95% limits of agreement − 15.06 to 13.51 mmHg). In regard to PETCO2 and PaCO2 difference, the value was − 2.10 mmHg (± 8.39) (95% − 18.54 to 14.33 mmHg) in all subjects. There was a higher correlation between PaCO2 and PTCCO2 values when compared to PaCO2 and PETCO2 (respectively, r = 0.66, p < 0.001; r = 0.51, p < 0.001). Various other studies have also found better correlations between PaCO2 and PTCCO2 values (correlation coefficients between 0.83 and 0.99) [22,23,24,25]. The rather lower level of correlation in our study may be explained by the inclusion of only critically ill children who required endotracheal intubation, whereas, healthy patients may have demonstrated relatively stable levels throughout comparisons performed with different methods.
In practice, the differences in the range of non-invasive CO2 measurement methods should be within the acceptable range [2, 19]. Accordingly, our results show that PCO2 measurements within the predefined, clinically acceptable range of ± 4 mmHg could be achieved by PTCCO2, but not by PETCO2. The difference between PaCO2 and PTCCO2 was ≤ ± 4 mmHg in 662 measurements out of the complete set of 1118 values (59.2%) while the difference between PaCO2 and PETCO2 was ≤ ± 4 mmHg in 471 out of overall 1118 values (%42.1). In other studies with acceptable bias (3 to 4.5 mmHg), it was found that 29–55% of PETCO2 measurements and 61–83% PTCCO2 measurements were within the acceptable level of bias [13, 26, 27].
There are few studies comparing the accuracy of non-invasive CO2 measurement methods. Tobias-Meyer et al. [11] studied intubated subjects in the PICU and found that the mean bias between PETCO2 and PaCO2 was 6.84 mmHg (± 5.1), whereas the mean bias between PTCCO2 and PaCO2 was 2.3 mmHg (± 1.3). Transcutaneous CO2 monitoring is also used in subjects with spontaneous breathing or non-invasive mechanical ventilator support, in addition to its use in those with invasive mechanical ventilation. In a study of non-intubated subjects in spontaneous respiration, simultaneous PETCO2, PTCCO2 and PaCO2 measurements were performed and showed very high correlation values between PTCCO2 and PaCO2 (r = 0.97), while moderate correlation (r = 0.62) was observed between PETCO2 and PaCO2 values [28].
Another strength of the current investigation lies in the subgroup analysis, where CO2 monitoring techniques were performed similarly in subjects with regard to the presence or absence of PD. When compared with PETCO2, PTCCO2 has been shown to be equally as accurate in children with normal respiratory function (non-PD group). The mean differences observed in the comparison of both methods with PaCO2 values were found to be similar. This is in line with a recent investigation in mechanically ventilated subjects without parenchymal lung disease [29]. Therefore, it could be postulated that, even though PTCCO2 determination seems to be better overall, PETCO2 monitoring is sufficient and accurate in subjects receiving MV, particularly if pulmonary disease is not present.
In contrast, the differences between each method and PaCO2 values increased in the presence of PD; however, PTCCO2 values were much more accurate compared to PETCO2 values. The present and previous trials have clearly demonstrated that monitoring with PETCO2 poorly estimates PaCO2 in subjects with PD [9, 19, 30,31,32,33]. This is most often explained by ventilation-perfusion mismatching and dead-space ventilation, as these two factors are associated with inadequate gas exchange that cannot be identified via PETCO2 [34, 35]. Therefore, it is apparent that the results of PaCO2 measurements in such patients will result in a lower value relative to actual CO2 levels [36, 37].
Previous reports have shown that PaCO2 measurements tend to be higher than the corresponding PETCO2 measurements [19, 38, 39] and the presence of PD further increases the PaCO2 and PETCO2 measurement gradient [40]. The results of our study are similar to the literature. However, 95% ULA values of the PETCO2–PaCO2 gradient were determined in the range of 13.01–15.04 mmHg, and these results are quite high compared to the literature [19]. In diseases that cause hemodynamic instability, such as sepsis and shock, PETCO2 measurements tend to be higher than corresponding PaCO2 measurements [41]. High 95% ULA values in our study may be associated with the presence of patients with hemodynamic instability (such as shock and multi-trauma diagnoses) in our study, and the analysis of the highest number of measurement values in the literature so far (1118 pairs).
Although agreement was good between PTCCO2 and PaCO2, it was still limited; most possibly due to the characteristics of our patient group. We performed multiple linear regression analysis with PTCCO2-PaCO2 as the dependent variable to determine factors affecting differences between measurements. We found that increased body temperature (p < 0.001) is related with falsely high PTCCO2 values. Compared to previous studies, we had a higher number of measurements that demonstrated similar results, somewhat contrasting to previously published findings [17, 25]. Despite frequent measurement of body temperature in these critically ill patients and setting the sensor to appropriate temperature before measurements, it is still possible that the actual local pressure at the measurement site was different from patient to patient (especially since these were all critically ill patients),thereby causing differences in results. This hypothesis is directly related to the operating principle of the sensor [42].
In this study, inotropic index was found to affect the accuracy of PTCCO2 measurements. There are concerns about the accuracy of PTCCO2 in situations that may compromise CO2 washout from the tissue, such as poor skin perfusion and low cardiac output [36]. In the current study, increased inotropic index (p < 0.001) was related to higher PTCCO2 values. Although some investigators have suggested that shock does not affect PTCCO2 accuracy [12, 16, 17], others have confirmed that the gradient between PTCCO2 and PaCO2 increases as tissue perfusion decreases [43,44,45]. In our study, an objective marker (inotropic index) was used as a marker of shock, therefore, enabling more accurate analysis compared to other studies. We think that inotropic-induced vasoconstriction could be expected to reduce the accuracy of transcutaneous monitoring.
This is the first study to assess the associations between PTCCO2 – PaCO2 measurements with regard to their correlation to skin and subcutaneous adipose tissue thickness. While measurements were not affected by skin thickness (p = 0.57), they were significantly influenced by an increase in subcutaneous fat tissue thickness (p = 0.007). Several studies reported conflicting results regarding the influence of skin thickness by indirect estimation of body mass index (BMI) on the diffusion of CO2 to the skin and therefore the values of PTCCO2 [4, 16, 46, 47]. In our study, skin thickness and subcutaneous adipose tissue thickness (at sites of transcutaneous CO2 sensor placement) were measured directly by using ultrasonography—leading to comparisons based on actual measurements rather than estimates. Since we were not able to find such evaluations in previous studies, we believe our study adds important data to the existing literature pertaining to transcutaneous CO2 measurement. Based on the results of our study, we may speculate that local conditions at the site of sensor placement, including the skin-subcutaneous adipose tissue thickness and conductivity of the skin, are more important for PTCCO2 measurement than whole body composition. Similarly, local edema increases the distance over which CO2 molecules travel to the probe; therefore, it could affect PTCCO2 measurements.
The results from our analyses have important implications for how transcutaneous CO2 monitoring should be applied. No specific recommendations for a preferred site or sites are provided by manufacturers. Similarly, guidelines on transcutaneous CO2 monitoring from the American Association for Respiratory Care do not provide a recommendation for the optimal site to place a transcutaneous CO2 sensor [42]. In addition, transcutaneous CO2 measurement was obtained from three different locations (chest, thigh and abdomen) in current study. In accordance with the literature, it was found that the measurement locations do not affect the accuracy of PTCCO2 measurements [4].
Although we have reached a large series of mechanically ventilated pediatric subjects and maximum number of transcutaneous CO2 measurement in the literature, there are some limitations in the study. Firstly, transcutaneous CO2 measurements were obtained from three different body locations of the subjects at separate times. It would be possible to compare much more collected data by increasing the number of time-points for measurement, and possibly, the number of body locations. Secondly, no evaluation was made regarding the effects of the thickness of muscle tissue at the measurement site. Thirdly, we limited our study to the TCM4 Radiometer PTCCO2 monitor. It is possible that other monitors perform with higher or lower accuracy. Finally, in this study, we did not record ventilation tidal volumes during PETCO2 measurements. Particularly low tidal volumes that are not sufficient to flush the anatomic dead volume may result in gas samples that do not represent the alveolar gas status. This is quite often a cause of low PETCO2 measurements.
5 Conclusion
The PTCCO2 method has higher reliability than the PETCO2 method for non-invasive monitoring of PCO2 in children undergoing invasive MV. Especially in children with PD, it is more reliable than PETCO2. However, PTCCO2 measurement is affected by subcutaneous fat (adipose) tissue thickness, core body temperature and inotropic index. PTCCO2 cannot replace ABG analysis in mechanically ventilated pediatric subjects, but it may be very useful to define early changes in ventilation, ease clinical management, and reduce the number of invasive procedures performed for arterial blood sampling.
References
Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc. 2014;11(4):645–52.
Tobias JD. Transcutaneous carbon dioxide monitoring in infants and children. Paediatr Anaesth. 2009;19(5):434–44.
Urbano J, Cruzado V, López-Herce J, del Castillo J, Bellón JM, Carrillo Á. Accuracy of three transcutaneous carbon dioxide monitors in critically ill children. Pediatr Pulmonol. 2010;45(5):481–6.
Gorska K, Korczynski P, Maskey-Warzechowska M, Chazan R, Krenke R. Variability of transcutaneous oxygen and carbon dioxide pressure measurements associated with sensor location. Adv Exp Med Biol. 2015;858:39–46.
Bhende MS. End-tidal carbon dioxide monitoring in pediatrics: clinical applications. J Postgrad Med. 2001;47(3):215–8.
Siobal MS. Monitoring exhaled carbon dioxide. Respir Care. 2016;61(10):1397–416.
Severinghaus J. The current status of transcutaneous blood gas analysis and monitoring. Blood Gas News. 1998;7(2):4–9.
Franklin ML. Transcutaneous measurement of partial pressure of oxygen and carbon dioxide. Respir Care Clin N Am. 1995;1(1):119–31.
Berkenbosch JW, Lam J, Burd RS, Tobias JD. Noninvasive monitoring of carbon dioxide during mechanical ventilation in older children: end-tidal versus transcutaneous techniques. Anesth Analg. 2001;92(6):1427–31.
Berkenbosch JW, Tobias JD. Transcutaneous carbon dioxide monitoring during high-frequency oscillatory ventilation in infants and children. Crit Care Med. 2002;30(5):1024–7.
Tobias JD, Meyer DJ. Noninvasive monitoring of carbon dioxide during respiratory failure in toddlers and infants: end-tidal versus transcutaneous carbon dioxide. Anesth Analg. 1997;85(1):55–8.
Tobias JD, Wilson WR Jr, Meyer DJ. Transcutaneous monitoring of carbon dioxide tension after cardiothoracic surgery in infants and children. Anest Analg. 1999;88(3):531–4.
Tschupp A, Fanconi S. A combined ear sensor for pulse oximetry and carbon dioxide tension monitoring: accuracy in critically ill children. Anesth Analg. 2003;96(1):82–4.
Senn O, Clarenbach CF, Kaplan V, Maggiorini M, Bloch KE. Monitoring carbon dioxide tension and arterial oxygen saturation by a single earlobe sensor in Patients with critical illness or sleep apnea. Chest. 2005;128(3):1291–6.
Herrejon A, Inchaurraga I, Palop J, Ponce S, Peris R, Terradez M, Blanquer R. Usefulness of transcutaneous carbon dioxide pressure monitoring to measure blood gases in adults hospitalized for respiratory disease. Arch Bronconeumol. 2006;42(5):225–9.
Bendjelid K, Schutz N, Stotz M, Gerard I, Suter PM, Romand JA. Transcutaneous PCO2 monitoring in critically ill adults: clinical evaluation of a new sensor. Crit Care Med. 2005;33(10):2203–6.
Rodriguez P, Lellouche F, Aboab J, Buisson CB, Brochard L. Transcutaneous arterial carbon dioxide pressure monitoring in critically ill adult patients. Intensive Care Med. 2006;32(2):309–12.
Bolliger D, Steiner LA, Kasper J, Aziz OA, Filipovic M, Seeberger MD. The accuracy of non-invasive carbon dioxide monitoring: a clinical evaluation of two transcutaneous systems. Anaesthesia. 2007;62(4):394–9.
Schwarz SB, Windisch W, Magnet FS, Schmoor C, Karagiannidis C, Callegari J, Huttmann SE, Storre JH. Continuous non-invasive PCO2 monitoring in weaning patients: transcutaneous is advantageous over end-tidal PCO2. Respirology. 2017;22(8):1579–84.
Urbano J, Cruzado V, Lopez-Herce J, del Castillo J, Bellon JM, Carrillo A. Accuracy of three transcutaneous carbon dioxide monitors in critically ill children. Pediatr Pulmonol. 2010;45(5):481–6.
DesPrez K, McNeil JB, Wang C, Bastarache JA, Shaver CM, Ware LB. Oxygenation saturation index predicts clinical outcomes in ARDS. Chest. 2017;152(6):1151–8.
Storre JH, Steurer B, Kabitz H-J, Dreher M, Windisch W. Transcutaneous PCO2 monitoring during initiation of noninvasive ventilation. Chest. 2007;132(6):1810–6.
McVicar J, Eager R. Validation study of a transcutaneous carbon dioxide monitor in patients in the emergency department. Emerg Med J. 2009;26(5):344–6.
Cox M, Kemp R, Anwar S, Athey V, Aung T, Moloney E. Non-invasive monitoring of CO2 levels in Patients using NIV for AECOPD. Thorax. 2006;61(4):363–4.
Bobbia X, Claret PG, Palmier L, Robert M, Grandpierre RG, Roger C, Ray P, Sebbane M, Muller L, La Coussave JE. Concordance and limits between transcutaneous and arterial carbon dioxide pressure in emergency department Patients with acute respiratory failure: a single-center prospective observational study. Scand J Trauma Resusc Emerg Med. 2015;17(23):40.
McBride DS Jr, Johnson JO, Tobias JD. Noninvasive carbon dioxide monitoring during neurosurgical procedures in adults: end-tidal versus transcutaneous techniques. South Med J. 2002;95(8):870–4.
Nosovitch MA, Johnson JO, Tobias JD. Noninvasive intraoperative monitoring of carbon dioxide in children: endtidal versus transcutaneous techniques. Pediatr Anesth. 2002;12(1):48–52.
Lermuzeaux M, Meric H, Sauneuf B, Girard S, Normand H, Lofaso F, Terzi N. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: a pilot study. J Crit Care. 2016;31(1):150–6.
Orlikowski D, Prigent H, Ambrosi X, Vaugier I, Pottier S, Annane D, Lofaso F, Ogna A. Comparison of ventilator-integrated end-tidal CO2 and transcutaneous CO2 monitoring in home-ventilated neuromuscular patients. Respir Med. 2016;117:7–13.
Hinkelbein J, Floss F, Denz C, Krieter H. Accuracy and precision of three different methods to determine PCO2 (PaCO2 vs. PETCO2 vs. PTCCO2) during interhospital ground transport of critically ill and ventilated adults. J Trauma. 2008;65(1):10–8.
Belpomme V, Ricard-Hibon A, Devoir C, Dileseigres S, Devaud ML, Chollet C, Marty J. Correlation of arterial PCO2 and PETCO2 in prehospital controlled ventilation. Am J Emerg Med. 2005;23(7):852–9.
Johnson DC, Batool S, Dalbec R. Transcutaneous carbon dioxide pressure monitoring in a specialized weaning unit. Respir Care. 2008;53.(8):1042–7.
Morley TF, Giaimo J, Maroszan E, Bermingham J, Gordon R, Griesback R, Zappasodi SJ, Giudice JC. Use of capnography for assessment of the adequacy of alveolar ventilation during weaning from mechanical ventilation. Am Rev Respir Dis. 1993;148(2):339–44.
Lumb A. Nunn's applied respiratory physiology. 5th ed. Oxford: Butterworth-Heinemann; 1999.
Krauss B, Deykin A, Lam A, Ryoo JJ, Hampton DR, Schmitt PW, Falk JL. Capnogram shape in obstructive lung disease. Anesth Analg. 2005;100(3):884–8.
Bhalla AK, Khemani RG, Hotz JC, Morzov RP, Newth CJ. Accuracy of transcutaneous carbon dioxide levels in comparison to arterial carbon dioxide levels in critically Ill children. Respir Care. 2019;64(2):201–8.
Rees SE, Larraza S, Dey N, Spadaro S, Brohus J, Winding RW, Volta CA, Kaerbing DS. Typical patterns of expiratory flow and carbon dioxide in mechanically ventilated Patients with spontaneous breathing. J Clin Monit Comput. 2017;31(4):773–81.
Baudin F, Bourgoin P, Brossier D, Essouri S, Emeriaud G, Wysocki M, Jouvet P. Noninvasive estimation of ARTERIAL CO2 from end-tidal CO2 in mechanically ventilated children: the GRAeDIENT pilot study. Pediatr Crit Care Med. 2016;17(12):1117–23.
Kugelman A, Zeiger-Agnsky D, Bader D, Shoris I, Riskin A. A novel method of distal end-tidal CO2 capnography in intubated infants: comparison with arterial CO2 and with proximal mainstream end-tidal CO2. Pediatrics. 2008;122(6):e1219–1224.
Yousuf T, Brinton T, Murtaza G, Woznicka D, Ahmad K, Iskandar J, Mehta R, Keshmiri H, Hanif T. Establishing a gradient between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide in patients with acute respiratory distress syndrome. J Investig Med. 2017;65(2):338–41.
Shetty A, Sparenberg S, Adams K, Selvedran S, Tang B, Hanna K, Iredell J. Arterial to end-tidal carbon dioxide tension difference (CO2 gap) as a prognostic marker for adverse outcomes in emergency department patients presenting with suspected sepsis. Emerg Med Australas. 2018;30(6):794–801.
Restrepo RD, Hirst KR, Wittnebel L, Wettstein R. AARC clinical practice guideline: transcutaneous monitoring of carbon dioxide and oxygen. Respir Care. 2012;57(11):1955–62.
Hillier SC, Badgwell JM, McLeod ME, Creighton RE, Lerman J. Accuracy of end-tidal PCO2 measurements using a sidestream capnometer in infants and children ventilated with the Sechrist infant ventilator. Can J Anaesth. 1990;37(3):318–21.
Tobias JD, Flanagan J, Wheeler TJ, Garrett JS, Burney C. Noninvasive monitoring of end-tidal CO2 via nasal cannulas in spontaneously breathing children during the perioperative period. Crit Care Med. 1994;22(11):1805–8.
Sivan Y, Eldadah MK, Te C, Newth CJ. Estimation of arterial carbon dioxide by end-tidal and transcutaneous PCO2 measurements in ventilated children. Pediatr Pulmonol. 1992;12(3):153–7.
Maniscalco M, Zedda A, Faraone S, Carratù P, Sofia M. Evaluation of a transcutaneous carbon dioxide monitor in severe obesity. Int Care Med. 2008;34(7):1340–4.
Cuvelier A, Grigoriu B, Molano LC, Muir JF. Limitations of transcutaneous carbon dioxide measurements for assessing long-term mechanical ventilation. Chest. 2005;127(5):1744–8.
Acknowledgements
The authors thank the nursing staff of the Pediatric Intensive Care Unit of Medeniyet University Goztepe Training and Research Hospital for their contributions to the conduct of this study, and the children and their families included in this study. The authors are indebted to all the ICU physicians who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The study protocol was approved by the Clinical Research Ethics Committee of Istanbul Medeniyet University Goztepe Training and Research Hospital (study registration number: 2017-9375).
Informed Consent
Informed consent was obtained from parents or legal guardians of children.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duyu, M., Mocan Çağlar, Y., Karakaya, Z. et al. Comparison of arterial CO2 estimation by end-tidal and transcutaneous CO2 measurements in intubated children and variability with subject related factors. J Clin Monit Comput 35, 101–111 (2021). https://doi.org/10.1007/s10877-020-00569-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00569-w