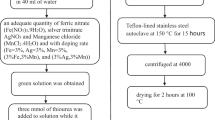
Abstract
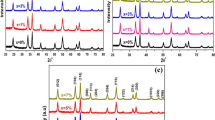
Undoped, Fe, Sn doped and Fe/Sn co-doped copper oxides are prepared by precipitation synthesis. X-ray diffraction confirmed that the all synthesized powders show a monoclinic main CuO phase. The insertion of Fe or/and Sn within the CuO matrix moderately affects the preferential growth direction. Basically Fourier Transform Infrared (FTIR) confirmed about functional group and their vibrations in the CuO samples. The reflectance of the undoped sample is higher than that of the other samples. Compared to undoped CuO, the doped and co-doped NPs exhibit red-shifted gap energy. Indeed, the Fe-doped, and Sn-doped CuO NPs, exhibit a slight decrease of gap energy (1.47, 1.45 eV respectively) compared to the undoped CuO (1.49 eV), while the Fe/Sn co-doped sample has a lower gap energy of 1.06 eV.
Additionally, the antibacterial efficiencies of the all-synthesized samples are tested against Staphyloccus species. Doped and undoped CuO nanopowders show important antibacterial activity on tested bacteria with MICs values ranged between 0.039 to 1.25 mg/ml. Minimum Inhibitory Concentration value of 0.039 mg/ml was obtained with Fe-doped CuO NPs (CuO:Fe NPs) against S.aureus ATCC33591, whereas the highest MIC value of 1.25 mg/ml was obtained with CuO:Sn nanopowder against the strain S. epidermidis, which was the most resistant strain. Moreover, all CuO NPs, except CuO:Fe/Sn showed important anti-adhesive and antibacterial activities against S. epidermidis when used as pellets. This was confirmed either by cell counts using the determination of CFU/ml of bacterial suspension inside the hole, or by using fluorescence microscopy.


















Similar content being viewed by others
Data Availability
No supplementary data, all data are presented in the manuscript.
References
H. N. Cuong, S. Pansambal, S. Ghotekar, R. Oza, N. T. T. Hai, N. M. Viet, and V. Nguyen (2022). A review Environ Res 203, 111858. https://doi.org/10.1016/j.envres.2021.111858.
M. E. Davey and G. A. O. Toole (2000). Microbiology and Molecular Biology Reviews 64 (4), 847–867. https://doi.org/10.1128/MMBR.64.4.847-867.2000).
G. Ren, D. Hu, E. W. C. Cheng, M. A. Vargas-Reus, P. Reip, and R. P. Allaker (2009). International Journal of Antimicrobial Agents 33 (6), 587–590.
Y.-W. Baek and Y.-J. An (2011). The Science of the Total Environment 409 (8), 1603–1608. https://doi.org/10.1016/j.scitotenv.2011.01.014.
A.-P. Magiorakos, A. Srinivasan, R. B. Carey, Y. Carmeli, M. E. Falagas, C. G. Giske, S. Harbarth, J. F. Hindler, G. Kahlmeter, B. Olsson-Liljequist, D. L. Paterson, L. B. Rice, J. Stelling, M. J. Struelens, A. Vatopoulos, J. T. Weber, and D. L. Monnet (2012). Clinical Microbiology and Infection 18 (3), 268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
T.C. Horan , R.P. Gaynes (2004). Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control, (3rd ed. Lippincott Williams & Wilkins; Philadelphia), p. 1659–1702
L. A. Grohskopf, R. L. Sinkowitz-Cochran, D. O. Garrett, A. H. Sohn, G. L. Levine, J. D. Siegel, B. H. Stover, and W. R. Jarvis (2002). J Pediatr 140, 432–438. https://doi.org/10.1067/mpd.2002.122499.
N. L. Rosi and C. A. Mirkin (2005). Chem Rev 105, 1547–1562. https://doi.org/10.1021/cr030067f.
KR. Raghupati, RT. KOOdali, AC. Manna (2011). Langmuir, 27, 4020-4028. doi.org/https://doi.org/10.1021/la104825u.
K. Lewis and A. M. Klibanov (2005). Trends Biotechnol 23, 343–348. https://doi.org/10.1016/j.tibtech.2005.05.004.
S. Saleem, A. H. Jabbar, M. H. Jameel, A. Rehman, Z. H. Kareem, A. H. Abbas, Z. Ghaffar, S. A. Razzaq, R. A. Pashameah, E. Alzahrani, Eng-Poh Ng, and S. M. Sapuan (2022). Nanotechnology Reviews 11, 2827–2838. doi.org/https://doi.org/10.1515/ntrev-2022-0473.
S. Saleem, M. Irfan ,M. Y. Naz, S. Shukrullah, M. A. Munir, M. Ayyaz, A. S. Alwadie, S. Legutko, J. Petrů and S. Rahman (2022). materials 15 (10), 3502. doi.org/https://doi.org/10.3390/ma15103502.
N. Tran, A. Mir, D. Mallik, A. Sinha, S. Nayar, and T. J. Webster (2010). Int J Nanomedecine 5, 277–283. https://doi.org/10.2147/ijn.s9220.
G. Applerot, J. Lellouche, A. Lipovsky, Y. Nitzan, R. Lubart, A. Gedanken, and E. Banin (2012). Small 8 (21), 3326–3337. https://doi.org/10.1002/smll.201200772.
Q. Zhang, K. Zhang, D. Xu, G. Yang, H. Huang, F. Nie, C. Liu, and S. Yang (2014). Prog. in Mater Sc 60, 208–337. https://doi.org/10.1016/j.pmatsci.2013.09.003.
O. Mahapatra, M. Bhagat, C. Gopalakrishnan, and K. D. Arunachalam (2008). J ExpNanosci 3, 185–193. https://doi.org/10.1080/17458080802395460.
A. Bhattacharjee and M. Ahmaruzzaman (2016). RSC Adv 6 (47), 41348–41363. https://doi.org/10.1039/C6RA03624D.
H. Qamar, S. Rehman, D. K. Chauhan, A. K. Tiwari, V. Upmanyu, (2020), Inter.J.Nanomed, 15, 2541–2553. https://doi.org/10.2147/IJN.S240232
P. Bhavyasree and T. Xavier (2022). Curr. Res. Green Sustainable Chem 5, 100249. https://doi.org/10.1016/j.crgsc.2021.100249
C. Tijo, A. Khursheed, S. Quaiser, M. Faisal, W. Rizwan, and M. Javed (2020). Biomolecules 10, 169. https://doi.org/10.3390/biom10020169.
V. Gnanavel, V. Palanichamy, and S. M. Roopan (2017). J. Photochem. Photobiol. B 171, 133–138. https://doi.org/10.1016/j.jphotobiol.2017.05.001.
M. Ahamed, H. A. Alhadlaq, M. A. Majeed Khan, Ponmurugan Karuppiah, and Naif A. Al-Dhabi (2014), J. Nanomater. https://doi.org/10.1155/2014/637858.
N. Khlifi, S. Mnif, F. Ben Nasr, N. Fourati, C. Zerrouki, M. M. Chehimi, H. Guermazi, S. Aifa, and S. Guermazi (2022). RSC Adv 12, 23527–23543. https://doi.org/10.1039/D2RA02433K.
A. Pugazhendhi, S. S. Kumar, M. Manikandan, and M. Saravanan (2018). Microbial Pathogenesis. https://doi.org/10.1016/j.micpath.2018.06.016.
P. Venkateswaria, P. Thirunavukkarasua, T. Sivakumar, and G. Sankar (2019). IOSR J. of Eng 9, 51–60.
S. Saleem, M. H. Jameel, A. A. Alothman, M. Z. Hilmi, B. Mayzan, T. Yousaf, M. R. Ahmad, A. Ali, and A. Zaman (2023). J Sol-Gel Sci and Tech. https://doi.org/10.1007/s10971-023-06287-4.
M. Fterich, F. Ben Nasr, R. Lefi, M. Toumi, and S. Guermazi (2016). Mater. Sci Semicond Process 43, 114–122. https://doi.org/10.1016/j.mssp.2015.11.023.
C. Satari, R. S. Sidqi, R. F. Putra, S. R. Putri, A. B. DaniNandiyanto (2021). Inter J.Energetica (IJECA), 6, 21-34. https://www.ijeca.info.
J.M. Simard (2007). Synthesis of gold nanoparticles for biomacromolecular recognition (Doctoral Dissertations). https://scholarworks.umass.edu/dissertations/AAI3275799
M. R. Arefi and S. R. Zarchi (2012). International journal of Molecular Sciences 13, 4340–4350. https://doi.org/10.3390/ijms13044340.
J. Gross, S. Sayle, A. R. Karow, U. Bakowsky, and P. Garidel (2016). Eur. J. Pharm. Biopharm 104, 30–41. https://doi.org/10.1016/j.ejpb.2016.04.013.
M. Jardak, A. Atoissi, D. Msalbi, D. Atoui, B. Bouizgarne, G. Rigane, R. Ben Salem, S. Aifa, and S. Mnif (2022). Center Microbial Pathogenesis 164, 105449. https://doi.org/10.1016/j.micpath.2022.105449.
J. A. Bearden and A. Burr (1967). Rev. Mod. Phys 39, 125. https://doi.org/10.1103/RevModPhys.39.125.
K. Martin and G. McCarthy (1990). North Dakota State Univ, Fargo, ND, USA. ICDD Grant-in-Aid (1991). Structure. J. Solid State Chem 89, 184.
S. Singhal, J. Kaur, T. Namgyal, R. Sharma (2012). Phys. B, 407, 1223–1226. doi. org/https://doi.org/10.1016/j.physb.2012.01.103
M. Rabiei, A. Palevicius, A. Monshi, S. Nasiri, A. Vilkauskas, and G. Janusas (2020). Nanomater. 10, 1627. https://doi.org/10.3390/nano10091627.
G. K. Williamson and W. H. Hall (1953). Acta Metall 1, 22–31. https://doi.org/10.1016/0001-6160(53)90006-6.
S. Kahraman, H. A. Çetinkara, F. Bayansal, H. M. Çakmak, and H. S. Güder (2012). Philos. Mag 92, 2150–2163. https://doi.org/10.1080/14786435.2012.669064.
S. Muthukumaran and R. Gopalakrishnan (2012). Opt. Mater 34, 1946–1953.
A. T. Ravichandran, K. C. AtherineSiriyaPushpa, K. Ravichandran, K. Karthika, B. M. Nagabhushana, S. Mantha, and K. Swaminathan (2014). Superlattice. Microst 75, 533–542. https://doi.org/10.1016/j.spmi.2014.08.009.
S. Saleem, M. N. Ashiq , S. Manzoor , U. Ali, R. Liaqat, A. Algahtani, S. Mujtaba, V. Tirth, A. M. Alsuhaibani, M. S. Refat, A. Ali, M. Aslam, A. Zaman (2023). Journal of materials research and technology, 25, 6150-6166. https://doi.org/10.1016/j.jmrt.2023.07.065
B. K. Ku, P. Kulkarni (2012). J. Aerosol Sci, 47, 100–110. https://doi.org/10.1016/2Fj.jaerosci.2012.01.002
L. Dörner, C. Cancellieri, B. Rheingans, M. Walter, R. Kägi, P. Schmutz, M. Kovalenko ,Lars P. H. Jeurgens (2019). Scientific Reports, 9, 11758. https://doi.org/10.1038/s41598-019-48020-8
O. P. Keabadile, A. O. Aremu, S. E. Elugoke and O. E. Fayemi (2020) Nanomater. https://doi.org/10.3390/nano10122502
M. U. AnnPrathap, B. Kaur, R. Srivastava (2012). J. Colloid Interface Sci, 381(1), 143-151. https: // doi .org/https://doi.org/10.1016/j.jcis.2012.05.025.
K. Ghenadii, H. Sang Do, C. Beongki, and T. Valeri (2009). Processing and Application of Ceramics 3 (1–2), 19–28.
S. Adil, A. Nafees, S. Saima, S. Suhail, and M. Zain Khan (2019). ACS Omega 4, 12905–12918. https://doi.org/10.1021/acsomega.9b01261.
P. K. Raul, S. Senapati, A. K. Sahoo, I. M. Umlong, Ri. R. Devi, A. J. Thakur, and V. Veer (2014). RSC Adv 4, 40580. https://doi.org/10.1039/c4ra04619f.
N. Wang, H. caiHe, L. Han (2010). Appl. Surf. Sci, 256 (23), 7335-7338. https: // doi. org/https://doi.org/10.1016/j.apsusc.2010.05.029
N. Tounsi, A. Barhoumi, F. C. Akkari, M. Kanzari, H. Guermazi, S. Guermazi. (2015).Vacuum, 121, 9-17. https://doi.org/10.1016/j.vacuum.2015.07.011.
S. Sonia, I. Jose Annsi, P. Suresh Kumar, D. Mangalaraj, C. Viswanathan, and N. Ponpandian (2015). Mater. Lett. 144, 127–130. https://doi.org/10.1016/j.matlet.2015.01.026.
S. Landi Jr., I. R. Segundo, E. Freitas, M. Vasilevskiy, J. Carneiro, and C. J. Tavares (2022). Solid State Comm 341, 114573. https://doi.org/10.1016/j.ssc.2021.114573.
S. Fabbiyola, L. Kennedy, A. A. Dakhel, M. Bououdina, J. Vijaya, and T. Ratnaji (2016). Journal of Molecular Structure 1109, 89–96. https://doi.org/10.1016/j.molstruc.2015.12.071.
A. N. Banerjee, K. K. Chattopadhyay,D. Depla , S. Maheiu (2008). In: Reactive Sputter Deposition, (Springer-Verlag Berlin Heidelberg), p. 465.
J. Tauc (1974). Amorphous and Liquid Semiconductors, (Plenum Press, London & New York) https://doi.org/10.1007/978-1-4615-8705-7
H. Jdidi, N. Fourati, C. Zerrouki, L. Ibos, M. Fois, A. Guinault, W. Jilani, S. Guermazi, and H. Guermazi (2021). Polymer 228, 123882. https://doi.org/10.1016/j.polymer.2021.123882.
P. Rani, A. Gupta, S. Kaur, V. Singh, S. Kumar, and Dinesh Kumar (2016). American Institute of Physics. 1728, id 020057. https://doi.org/10.1063/1.4946108.
F. Urbach (1953). J. Phys. Rev. 92, 1324. https://doi.org/10.1103/PhysRev.92.1324.
B. Abay, S. H. Güder, H. Efeoglu, and K. Y. Yogurtçu (2001). Turk. J. Phys 25, 543–549.
H. Mahr (1962). Phys. Rev 125, 1510. https://doi.org/10.1103/PhysRev.125.1510.
C. Gao, H. Shen, and L. Sun (2011). Applied Surface Science 257, 6750–6755. https://doi.org/10.1016/j.apsusc.2011.02.116.
G. N. S. Vijayakumar, M. Rathnakumari, and P. Sureshkumar (2011). Arch. Appl. Sci. Res 3, 514–525.
J. I. Pankove (1975). Optical Processes in Semiconductors, (Dover Publications, Inc, New York).
K. Takenaka, Y. Imanaka, K. Tamasaku, T. Ito, and S. Uchida (1992). Phys Rev B 46, 5833(R).
H. Weiwei, K. Hyun-Kyung, W. G. Wamer, M. David, J. H. Callahan, and Y. Jun-Jie (2014). J Am Chem Soc 136 (2), 750–757. https://doi.org/10.1021/ja410800y.
U. Kadiyala, A. Nicholas, J. S. Kotov, and V. Eppsa (2018). Curr Pharm Des, 24 (8), 896–903. https://doi.org/10.2174/1381612824666180219130659.
Ameer Azam, Arham S. Ahmed, M. Oves, M. S. Khan, and Adnan Memic (2012). Journal of Nanomedicine. 7, 3527–3535. https://doi.org/10.2147/ijn.s29020.
M. Amiri, Z. Etemadifar, A. Daneshkazemi, and M. Nateghi (2017). Antimicrobial Effect of Copper Oxide NPs on Some Oral Bacteria and Candida Species 4 (1), 347–352.
Munin Agarwala, Bula Choudhury, and R. N. S. Yadav (2014). Indian J Microbiol 54 (3), 365–368. https://doi.org/10.1007/s12088-014-0462-z.
A. Pugazhendhi, S. Kumar, M. Manikandan, and M. Saravanan (2018). Microbial pathogenesis 122, 84–89. https://doi.org/10.1016/j.micpath.2018.06.016.
Acknowledgements
Authors thank the financial support of the Tunisian Ministry of High Education and ScientificResearch.
Funding
This work was funded by Tunisian Ministry of High Education and Scientific Research
Author information
Authors and Affiliations
Contributions
F.B and H.G: writing and evaluation... S.M and S.A: Completion of biological experiments... B.D and G.L: characterization experiments... S.G: reviewed
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
We Participants in writing the manuscript declare thatthis manuscript is original, has not been publishedbefore and is not currently being considered forpublication elsewhere. We confirm that the manuscript has been read and approved by all namedauthors and that there are no other persons whosatisfied the criteria for authorship but are not listed.We further confirm that the order of authors listed inthe manuscript has been approved by all of us. Weunderstand that the Corresponding Author is the solecontact for the Editorial process. He is responsiblefor communicating with the other authors aboutprogress, submissions of revisions and final approvalof proofs.
Research involving Human Participants and/or Animals
No Human or animal participants are involved.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Nasr, F., Mnif, S., Guermazi, H. et al. Synthesis, Characterization of (Fe, Sn) Doped and Co-Doped Copper Oxide Nanoparticles and Evaluation of their Antibacterial Activities. J Clust Sci (2024). https://doi.org/10.1007/s10876-024-02613-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10876-024-02613-0