Abstract
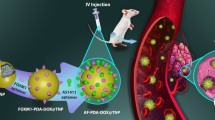
Active delivery of anti-cancer nanoparticles (NPs) represents a possibly influential approach, especially when treating hepatocellular carcinoma (HCC). We synthesized NPs decorated with Aptamer-AS1411 (APT), which selectively binds nucleolin on the surface of hepatoma cells. APT acts as an active targeting ligand that facilitates delivery of viramidine (VRM) against HCC cells. The conjugation between APT and NPs was confirmed by FTIR, 1H NMR, and TEM, and the resulting APT + VRM NPs were uniformly having a round shape with size of 141.77 ± 11.29 nm and zeta potential at − 43.71 ± 9.2 mV. Significant enhancement of cellular association between nucleolin and APT NPs was observed in Huh-7 more than HepG2 cells, thus, an increased cytotoxic effect against the first cell type over 2 days was seen. Furthermore, it was demonstrated that APT + VRM NPs arrest cancerous cells in G1 cell cycle phase, which is associated by a depletion in CDC25A expression. The downregulation of CDC25A is associated with overexpression of p53 and downregulation of PI3k, NF-κB, and STAT-1 expression in the nano-treated HCC cells compared with control. The outputs of protein levels showed the same pattern of the genetic expression levels of all candidates. The outcomes of this approach indicated the significant therapeutic value of APT + VRM NPs.





Similar content being viewed by others
Data Availability
Available and approved by all authors.
References
R. Siegle, D. Naishadham, and A. Jemal (2012). CA Cancer J. Clin. 62 (1), 10.
A. A. Abd-Rabou and H. H. Ahmed (2017). Adv. Med. Sci. 62 (2), 357.
M. G. Hart, et al. (2011). Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007294.pub2.
J. M. Pipas, et al. (2005). J. Neuro-Oncol. 71 (3), 301.
F. Alexis, et al. (2008). Mol. Pharm. 5 (4), 505.
L. Li, H. He, S. Jiang, et al. (2021). Molecules (Basel, Switzerland) 26 (5), 1271.
H. Xin, et al. (2011). Biomaterials 32 (18), 4293.
H. He, et al. (2011). Biomaterials 32 (2), 478.
S. A. Moosavian and A. Sahebkar (2019). Cancer Lett. 448, 144.
A. G. Hovanessian, et al. (2010). PloS One 5 (12), e15787.
J. Guo, et al. (2011). Biomaterials 32 (31), 8010.
H. Y. Ko, et al. (2011). Biomaterials 32, 1130.
E. M. Reyes-Reyes, Y. Teng, and P. J. Bates (2010). Cancer Res. 70 (21), 8617.
D. Legrand, et al. (2004). Eur. J. Biochem. 271 (2), 303.
X. Ji, C. Hou, et al. (2020). Food Funct. 11 (1), 163.
Y. Cheng, G. Zhao, et al. (2016). PLoS One 11 (12), e0167094.
J. Al-Matouq, T. R. Holmes, et al. (2019). Mol. Carcinog. 58 (9), 1691.
Y. Song, Z. Wang, et al. (2020). J. Cell Mol. Med. 24 (23), 13739.
A. C. Girvan, Y. Teng, et al. (2006). Mol. Cancer Ther. 5 (7), 1790.
M. Szelag, J. Wesoly, et al. (2016). Curr. Protein Pept. Sci. 17 (2), 135.
A. A. Abd-Rabou, D. J. Bharali, and S. A. Mousa (2020). Appl. Biochem. Biotechnol. 190 (1), 305.
A. A. Abd-Rabou, D. J. Bharali, and S. A. Mousa (2018). Pharm. Res. 35 (4), 1.
B. M. Rothen-Rutishauser, et al. (2006). Environ. Sci. Technol. 40 (14), 4353.
X. Gao, et al. (2006). Biomaterials 27 (18), 3482.
J. Cheng, et al. (2007). Biomaterials 28 (5), 869.
A. Aravind, et al. (2012). Biotechnol Bioeng 109 (11), 2920.
J. Van Meerloo, G. J. Kaspers, and J. Cloos, in Cancer Cell Culture (Springer, Berlin, 2011), pp 237–245.
M. Koutsioumpa, et al. (2013). J. Biol. Chem. 288 (1), 343.
H. Chen, et al. (2013). J. Biotechnol. 168 (4), 362.
C. F. Semenkovich, et al. (1990). Biochemistry 29 (41), 9708.
Y. Cho, et al. (2016). PloS One 11 (8), e0160822.
L. Zhao, X. Qi, et al. (2019). J. Am. Chem. Soc. 141 (44), 17493.
K. Y. Lee, et al. (2010). J. Biomed. Biotechnol. 2010, 1.
C. R. Ireson and L. R. Kelland (2006). Mol Cancer Ther. 5 (12), 2957.
E. W. Ng, et al. (2006). Nat. Rev. Drug Discov. 5 (2), 123.
A. Keefe, S. Pai, and A. Ellington (2010). Nat. Rev. Drug Discov. 9, 537.
M. Ahmed and R. Narain (2015). Nanomedicine 10 (14), 2263.
J. Varshosaz, et al. (2012). J. Liposome Res. 22 (3), 224.
D.-C. Li, et al. (2009). J. Controlled Release 138 (2), 103.
F. Marcucci and F. Lefoulon (2004). Drug Discov. Today 9 (5), 219.
B. Song, et al. (2009). Colloids Surf. B Biointerfaces 70 (2), 181.
Q. Gan, et al. (2005). Colloids Surf. B Biointerfaces 44 (2–3), 65.
M. Cheng, et al. (2012). PloS One 7, e47115.
K. Ninomiya, et al. (2013). Bioorg. Med. Chem. Lett. 23 (6), 1797.
D. Sun, et al. (2015). Anal. Chim. Acta 885, 166.
J. Yu, et al. (2020). Nanoscale Res. Lett. 15 (1), 1.
Y. Cho, et al. (2016). Dig. Dis. Sci. 61 (9), 2568.
F. Mongelard and P. Bouvet (2010). Curr. Opin. Mol. Ther. 12 (1), 107.
J. E. Rosenberg, et al. (2014). Investig. New Drugs 32 (1), 178.
B. E. Eaton, et al. (1997). Bioorg. Med. Chem. 5 (6), 1087.
A. A. Abd-Rabou and A. E. Edris (2021). Cancer Nanotechnol. 12 (1), 28.
A. A. Abd-Rabou, A. M. Abdelaziz, O. G. Shaker, and G. Ayeldeen (2021). Mol. Biol. Rep. 48 (10), 6805.
L. Chen, M. He, et al. (2021). Pharmacol. Ther. (Oxford) 226, 107868.
Funding
The authors declare that this publication was funded from National Research Centre to Ass. Prof. AA Abd-Rabou (Grant No. 12060129).
Author information
Authors and Affiliations
Contributions
All authors contributed in this study. AAA, the corresponding author, conceived the research idea, synthesized and characterized the NPs, as well as performed cell culture, cytotoxicity, flow cytometry, biochemical and genetic experiments, data analysis, wrote the manuscript, and responsible for publication. MSK and HHA participated in cytotoxicity and biochemical experiments. HHA shared in the analysis and editing the manuscript. KMMZ participated in the genetic and protein experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
The protocol of this article is ethically approved by the Ethical Committee for Medical Research, National Research Centre (Approval No. 19/200).
Consent to Participate
It is an in vitro study on cell lines.
Consent to Publish
Available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd-Rabou, A.A., Ahmed, H.H., Kishta, M.S. et al. Selective Viramidine-Loaded Aptamer-Nanoparticles Trigger Cell Cycle Arrest in Nucleolin-Expressed Hepatoma Cells Through Modulation of CDC25A/p53/PI3k Pathway. J Clust Sci 34, 335–348 (2023). https://doi.org/10.1007/s10876-022-02224-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02224-7