Summary
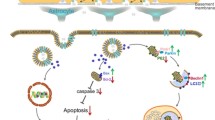
During the previous years, with the emerging of nanotechnology, the enormous capabilities of nanoparticles have drawn great attention from researchers in terms of their potentials in various aspects of pharmacology. Cerium oxide nanoparticles (nanoceria), considered as one of the most widely used nanomaterials, due to its tempting catalytic antioxidant properties, show a promising potential in diverse disorders, such as cerebral ischemic stroke (CIS), cancer, neurodegenerative and inflammatory diseases. Overwhelming generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during cerebral ischemia and reperfusion periods is known to aggravate brain damage via sophisticated cellular and molecular mechanisms, and therefore exploration of the antioxidant capacities of nanoceria becomes a new approach in reducing cerebral ischemic injury. Furthermore, utilizing nanoceria as a drug carrier might display the propensity to overcome limitations or inefficacy of other conceivable neuroprotectants and exhibit synergistic effects. In this review, we emphasize on the principle features of nanoceria and current researches concerning nanoceria as a potential therapeutic agent or carrier in improving the prognosis of CIS.
Similar content being viewed by others
References
Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke, 2011,42(8): 2351–2355
Celardo I, Traversa E, Ghibelli L. Cerium oxide nanoparticles: a promise for applications in therapy. J Exp Ther Oncol, 2011,9(1):47–51
Celardo I, Pedersen JZ, Traversa E, et al. Pharmacological potential of cerium oxide nanoparticles. Nanoscale, 2011,3(4):1411–1420
Wason MS, Colon J, Das S, et al. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine, 2013,9(4):558–569
Gao Y, Chen K, Ma JL, et al. Cerium oxide nanoparticles in cancer. Onco Targets Ther, 2014,7:835–840
Hirst SM, Karakoti AS, Tyler RD, et al. Antiinflammatory properties of cerium oxide nanoparticles. Small, 2009,5(24):2848–2856
Estevez AY, Pritchard S, Harper K, et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med, 2011,51(6):1155–1163
Kim CK, Kim T, Choi IY, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl, 2012,51(44):11039–11043
Heckman KL, DeCoteau W, Estevez A, et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano, 2013,7(12):10582–10596
Niu J, Azfer A, Rogers LM, et al. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res, 2007,73(3):549–559
Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 2016,47(2):581–641
Davis SM, DonnanGA. 4.5 hours: the new time window for tissue plasminogen activator in stroke. Stroke, 2009,40(6):2266–2267
Messé SR, Fonarow GC, Smith EE, et al. Use of tissuetype plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes, 2012,5(3):321–326
Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology, 2008,55(3): 310–318
Wang D, Yuan X, Liu T, et al. Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules, 2012,17(8):9803–9817
Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab, 2001,21(1):2–14
Mehta SH, Webb RC, Ergul A, et al. Neuroprotection by tempol in a model of iron-induced oxidative stress in acute ischemic stroke. Am J Physiol Regul Integr Comp Physiol, 2004,286(2):R283–288
Nakamura T, Kume T, Katsuki H, et al. Protective effect of serofendic acid on ischemic injury induced by occlusion of the middle cerebral artery in rats. Eur J Pharmacol, 2008,586(1-3):151–155
Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal, 2012,24(5):981–990
Gutteridge JM, Halliwell B. Comments on review of Free Radicals in Biology and Medicine, second edition, by Barry Halliwell and John M. C. Gutteridge. Free Radic Biol Med, 1992,12(1):93–95
Matsuda S, Umeda M, Uchida H, et al. Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. J Neural Transm, 2009,116(4):395–404
Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest, 2011,121(8):3005–3023
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets, 2013,12(5):698–714
Celardo I, De Nicola M, Mandoli C, et al. Ce(3)+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano, 2011,5(6):4537–4549
Neumann G, Hicks J. Effects of Cerium and Aluminum in Cerium-Containing Hierarchical HZSM-5 Catalysts for Biomass Upgrading. Topics in Catalysis, 2012,55(3-4):196–208
Korsvik C, Patil S, Seal S, et al. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb), 2007,10:1056–1058
Ganesana M, Erlichman JS, Andreescu S. Real-time monitoring of superoxide accumulation and antioxidant activity in a brain slice model using an electrochemical cytochrome c biosensor. Free Radic Biol Med, 2012,53(12):2240–2249
Pirmohamed T, Dowding JM, Singh S, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb), 2010,46(16):2736–2738
Dowding JM, Dosani T, Kumar A, et al. Cerium oxide nanoparticles scavenge nitric oxide radical (NO). Chem Commun (Camb), 2012,48(40):4896–4898
Xue Y, Luan QF, Yang D, et al. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C, 2011,115(11):4433–4438
Asati A, Santra S, Kaittanis C, et al. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed Engl, 2009,48(13):2308–2312
Wang Y, Yang F, Zhang HX, et al. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis, 2013,4:e783
Das M, Patil S, Bhargava N, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials, 2007,28(10):1918–1925
Schubert D, Dargusch R, Raitano J, et al. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun, 2006,342(1):86–91
Chen XM, Chen HS, Xu MJ, et al. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin, 2013,34(1):67–77
Pinzon-Daza ML, Campia I, Kopecka J, et al. Nanoparticle-and liposome-carried drugs: new strategies for active targeting and drug delivery across blood-brain barrier. Curr Drug Metab, 2013,14(6):625–640
Matoba T, Egashira K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int Heart J, 2014,55(4):281–286
Thompson BJ, Ronaldson PT. Drug delivery to the ischemic brain. Adv Pharmacol, 2014,71:165–202
Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. Faseb J, 2009,23(5):1384–1395
Yun X, Maximov VD, Yu J, et al. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab, 2013,33(4):583–592
Karakoti AS, Monteiro-Riviere NA, Aggarwal R, et al. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. JOM (1989), 2008,60(3):33–37
Wong LL, Hirst SM, Pye QN, et al. Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PLoS One, 2013,8(3):e58431
Zhou X, Wong LL, Karakoti AS, et al. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PLoS One, 2011,6(2):e16733
Cimini A, D'Angelo B, Das S, et al. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Abeta aggregates modulate neuronal survival pathways. Acta Biomater, 2012,8(6):2056–2067
Pagliari F, Mandoli C, Forte G, et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano, 2012,6(5):3767–3775
Lin W, Huang YW, Zhou XD, et al. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol, 2006,25(6):451–457
Alili L, Sack M, Karakoti AS, et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials, 2011,32(11): 2918–2929
Karakoti AS, Satyanarayana VNTK, Suresh Babu K, et al. Direct Synthesis of Nanoceria in Aqueous Polyhydroxyl Solutions. J Phys Chem C, 2007,111(46):17232–17240
Portioli C, Benati D, Pii Y, et al. Short-term biodistribution of cerium oxide nanoparticles in mice: focus on brain parenchyma. Nanosci Nanotechnol Lett, 2013,5(11): 1174–1181
Ma JY, Mercer RR, Barger M, et al. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology, 2011,5(3):312–325
Ma JY, Zhao H, Mercer RR, et al. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol Appl Pharmacol, 2012,262(3):255–264
Perez JM, Asati A, Nath S, et al. Synthesis of biocompatible dextran-coated nanoceria with pHdependent antioxidant properties. Small, 2008,4(5):552–556
Asati A, Santra S, Kaittanis C, et al. Surface-chargedependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano, 2010,4(9):5321–5331
Karakoti AS, Munusamy P, Hostetler K, et al. Preparation and Characterization Challenges to Understanding Environmental and Biological Impacts of Nanoparticles. Surf Interface Anal, 2012,44(5):882–889
Dowding JM, Das S, Kumar A, et al. Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano, 2013,7(6):4855–4868
Patil S, Reshetnikov S, Haldar MK, et al. Surface-Derivatized Nanoceria with Human Carbonic Anhydrase II Inhibitors and Fluorophores: A Potential Drug Delivery Device. J Phys Chem C, 2007,111(24):8437–8442
Vincent A, Babu S, Heckert E, et al. Protonated nanoparticle surface governing ligand tethering and cellular targeting. ACS Nano, 2009,3(5):1203–1211
Li M, Shi P, Xu C, et al. Cerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer's disease treatment. Chem Sci, 2013,4(6):2536–2542
Bi JJ, Yi L. Effects of integrins and integrin alphavbeta3 inhibitor on angiogenesis in cerebral ischemic stroke. J Huazhong Univ Sci Technolog Med Sci, 2014,34(3):299–305
Shimamura N, Matchett G, Yatsushige H, et al. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke, 2006,37(7):1902–1909
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, D., Fang, T., Lu, Lq. et al. Neuroprotective potential of cerium oxide nanoparticles for focal cerebral ischemic stroke. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 480–486 (2016). https://doi.org/10.1007/s11596-016-1612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1612-9