Abstract
Purpose
Asymptomatic SARS-CoV-2 infections were widely reported during the COVID-19 pandemic, acting as a hidden source of infection. Many existing studies investigating asymptomatic immunity failed to recruit true asymptomatic individuals. Thus, we conducted a longitudinal cohort study to evaluate humoral- and cell-mediated responses to infection and vaccination in well-defined asymptomatic young adults (the Asymptomatic COVID-19 in Education [ACE] cohort).
Methods
Asymptomatic testing services located at three UK universities identified asymptomatic young adults who were subsequently recruited with age- and sex-matched symptomatic and uninfected controls. Blood and saliva samples were collected after SARS-CoV-2 Wuhan infection, and again after vaccination. 51 participant’s anti-spike antibody titres, neutralizing antibodies, and spike-specific T-cell responses were measured, against both Wuhan and Omicron B.1.1.529.1.
Results
Asymptomatic participants exhibited reduced Wuhan-specific neutralization antibodies pre- and post-vaccination, as well as fewer Omicron-specific neutralization antibodies post-vaccination, compared to symptomatic participants. Lower Wuhan and Omicron-specific IgG titres in asymptomatic individuals were also observed pre- and post-vaccination, compared to symptomatic participants. There were no differences in salivary IgA levels. Conventional flow cytometry analysis and multi-dimensional clustering analysis indicated unvaccinated asymptomatic participants had significantly fewer Wuhan-specific IL-2 secreting CD4+ CD45RA+ T cells and activated CD8+ T cells than symptomatic participants, though these differences dissipated after vaccination.
Conclusions
Asymptomatic infection results in decreased antibody and T cell responses to further exposure to SARS-CoV-2 variants, compared to symptomatic infection. Post-vaccination, antibody responses are still inferior, but T cell immunity increases to match symptomatic subjects, emphasising the importance of vaccination to help protect asymptomatic individuals against future variants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2020, the World Health Organisation declared the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), outbreak a global pandemic, subsequently accounting for an estimated 18.2 million deaths worldwide between January 2020 and December 2021 [1]. The high rate of transmission and infection was the catalyst of the pandemic, resulting in the implementation of infection control measures, such as the isolation of individuals experiencing symptoms [2]. The proportion of those with no symptoms during SARS-CoV-2 infection, termed asymptomatic, was estimated to be 20-44% of COVID-19 cases [3,4,5,6,7], with increased cases in children to young adults, but decreased asymptomatic cases at older ages [7]. With a high frequency of asymptomatic cases, in addition to symptomatic individuals transmitting SARS-CoV-2 before the onset of symptoms, SARS-CoV-2 was transmitted silently, exacerbating the pandemic.
The assessment of the immune response to SARS-CoV-2 infection has primarily focused on spike-specific antibody responses or neutralising titres [8, 9]; SARS-CoV-2-infected individuals display neutralising antibodies for months or even years, facilitating these measurements [10]. The challenges of cellular assays, and logistics of obtaining suitable samples, has led to fewer studies focused on measuring specific T cell responses to SARS-CoV-2. T cells regulate antibody (humoral) responses, but also mediate a faster and more potent response upon further exposure to viral antigens, underpinning a long-lasting immunity and vaccine efficacy [11]. Indeed, there is evidence that cognate T cell responses are a better indicator of immunity than antibody levels [12]. T cell memory after SARS-CoV-2 infection is established with robust CD4+ and CD8+ T cell responses to a combination of spike, membrane, and nucleocapsid viral proteins [13], or spike-specific responses after vaccination.
The question of whether humoral and/or cellular immunity following asymptomatic SARS-CoV-2 infection is sufficient to protect from future strains compared to symptomatic infection is uncertain. Existing evidence suggests asymptomatic infection results in a faster decline of SARS-CoV-2-specific T cells compared to symptomatic infection [14], indicating a relationship between increased memory T cells and symptomatic disease. However, there is also evidence for a more robust SARS-CoV-2-specific early T cell-mediated response in asymptomatic than symptomatic patients, but a weaker neutralising antibody profile [15, 16].
Developing an understanding of asymptomatic and symptomatic immune responses to SARS-CoV-2 is imperative in minimising the future impact of another public health threat, such as through the development of T cell-targeted vaccines for any future strains of SARS-CoV-2 that may develop. In addition, understanding cross-reactivity to new variants is key for planning for potential vaccine strategies. This study took advantage of an on-going asymptomatic screening service developed at three universities in the UK, allowing measurements of T cell and serological responses to SARS-CoV-2 in individuals with true asymptomatic infection. These samples enabled us to build upon existing research and understand for the first time the immune responses in this asymptomatic, largely young, healthy demographic, with matched symptomatic subjects, following SARS-CoV-2 natural infection and subsequent vaccination. Utilising this genuine asymptomatic cohort, we identify key decreases in both humoral and cellular immune responses to SARS-CoV-2 in asymptomatic infection compared to symptomatic infection.
Methods
Study Design and Cohort
A multi-centre longitudinal cohort study was conducted in individuals identified as part of three University Asymptomatic Testing Services (The University of Nottingham, The University of Cambridge and Cardiff University). Eligible participants were aged 18 years or older. Participants were excluded if they were under 18 years old, unable to provide blood samples, had low English proficiency, unable to travel for the study visits, or unable to provide informed consent. Young adults who experienced no symptoms but were identified as positive for SARS-CoV-2 infection (asymptomatic) by one of the asymptomatic testing services, utilising antibody and PCR tests, were recruited into the study. Age and sex-matched participants, who were either negative for SARS-CoV-2 infection (had no symptoms and a negative test result for SARS-CoV-2), or who had symptomatic infection (experienced symptoms of SARS-CoV-2 infection and received a positive test result) were also recruited into the study.
Data and Sample Collection
A baseline questionnaire including information on demographics, clinical factors (previous COVID-19 infection and tests), and vaccination details was completed. Blood samples to isolate peripheral blood mononuclear cells (PBMCs) and plasma, as well as saliva samples, were then collected from all participants in Spring 2021, pre-vaccination, and a minimum of 1-week post-infection for asymptomatic and symptomatic participants. The participants then returned in Summer 2021, post-vaccination for a second blood and saliva collection. Whole blood samples were collected into heparin vacutainers (Greiner Bio-One) and within 4 hours they were diluted with an equal volume of PBS + 1% foetal bovine serum (FBS) and separated by density-gradient centrifugation, using Histopaque solution (Sigma) with SepMate tubes (Stem cell Technologies). PBMCs were washed twice and then immediately cryopreserved at -80°C in 10% DMSO and 90% FBS at a cooling rate of 1°C/min and subsequently transferred to liquid nitrogen storage (-196°C) until experimental use. Saliva samples were collected by spitting into a tube which contained a 2% final concentration Triton X-100 and stored at -80°C until use in IgA antibody ELISAs.
Antibody Titre Assay
Heparinized whole blood was centrifuged at 300 xg for 8 minutes and the upper plasma containing layer removed and further centrifuged at 800 xg for 5 minutes. Plasma was tested using two separate ELISAs for detection of Wuhan and Omicron-specific spike antibodies. Full methods can be found in supplementary methods.
Salivary IgA Assay
Before performing ELISAs, saliva samples were centrifuged at 1200 rpm for 1 minute, and a visual quality control step was performed, removing samples with excess debris present. IgA ELISAs were then performed (details found in supplementary methods), with samples diluted 1:15 in 3% whey blocking solution, replacing gamma-chain specific anti-human IgG HRP conjugate with an alpha-chain specific anti-human IgA HRP conjugate (Sigma, A0295), at a 1:10,000 dilution.
Generation of Pseudotyped Lentiviral Particles
Assays were performed as previously described [17]. In brief, Wuhan and Omicron pseudotyped viruses were generated using triple plasmid transfection of HEK293T cells with spike-expressing plasmid along with the lentiviral packaging vector p8.91 and luciferase expression vector psCSFLW using Lipofectamine transfection reagent (Thermo-Fisher). Lentiviruses were harvested after 48 h, passed through a 0.45μm filter, stored in aliquots at -80°C and titrated on HeLa cells expressing ACE-2 (HeLa-ACE2).
Virus Neutralization Assays
As shown previously [17], pseudotyped viruses were neutralized by incubating with serially diluted, heat-inactivated human plasma samples for 1 h at 37°C. Full methods can be found in supplementary methods.
T Cell Stimulation With Overlapping Peptide Pools
Cryopreserved PBMCs were thawed, washed, and resuspended in RPMI1640 10% FBS, 10U/mL benzonase (1 hr, 37C). Cells were washed and resuspend at 8x106 cells/ml in RPMI + 5% human AB serum in 96 well plates. PBMCS were stimulated with 0.6nM (~1mg/ml) SARS-CoV-2 overlapping peptide pools for Wuhan (Miltenyi) or Omicron (B.1.1.529.1) (Proimmune) spike. As positive controls cells were stimulated with 50 ng/mL Phorbol myristate acetate (PMA) and 0.5 μg/mL Ionomycin (for ICS assay), or 1 μg/mL Phytohemagglutinin (PHA) (for activation-induced marker (AIM) assay). As peptide pools were reconstituted in high-grade pure sterile H2O, cells incubated with a matched volume of H20 were used as a negative control. PBMCs were then split into either the AIM assay or ICS assay below.
Activation-Induced Marker Assay
An AIM assay was used to identify total CD4+ and CD8+ T cells responding to spike. cells were stimulated with peptides for 24 hours to allow adequate activation and stained with viability dye and surface markers (Table 1, 30 mins 4 °C). Cells were washed and resuspended in 4% paraformaldehyde before analysis on a Sony ID7000 spectral cell analyzer. The gating strategies for the AIM assay can be found in supplementary Figure 1.
Intracellular Staining Assay
An intracellular staining (ICS) assay was performed to determine the memory phenotype and cytokine profile of sars-co2 specific T-cells. For the ICS assay, 1x protein transport inhibitor cocktail (Thermofisher) was added after two hours of peptide stimulation and cells were cultured for an additional 4 hours (37°C, 5% CO2). A 21-colour flow cytometry panel was used (Table 2). Surface markers were stained for 30 minutes at 4 °C), then washed and fixed with 4% paraformaldehyde (15mins, 4°C), before being washed and permeabilized with permeabilization wash buffer Cells were then stained for intracellular cytokines in the presence of permeabilization wash buffer (30 mins, RT). Cells were then washed and fixed with 4% paraformaldehyde before analysis on a Sony ID7000 spectral cell analyzer.
The gating strategies for the ICS assay can be found in supplementary Figure 2.
Multi-dimensional Clustering Analysis
To fully elucidate the differences between phenotypes of spike specific T-cells between asymptomatic and symptomatic infection, multi-dimensional clustering analysis was performed. Single, viable, CD3+/CD14-/CD19- T-cells from spike peptide-stimulated PBMCS were gated in FlowJo (V.10) and 68 thousand T-cells down sampled per donor. T-cells from 19 donors within each group (asymptomatic unvaccinated, asymptomatic vaccinated, symptomatic unvaccinated, and symptomatic vaccinated) were concatenated and cytokine producing cells gated for by unsupervised analysis. For visualization, T-distributed stochastic-neighbour embedding (t-SNE) was performed with opt-SNE learning configuration, 1000 iterations, a perplexity of 30, learning rate of 3500, Exact (vantage point tree) KNN and Barnes-Hut gradient algorithm [18]. FlowSOM clustering [19] was performed using 28 meta-clusters for Wuhan analysis, and 27 meta-clusters for Omicron analysis, and projected on the t-SNE using Cluster Explorer.
Statistical Analysis
Details on data analysis can be found in the supplementary methods.
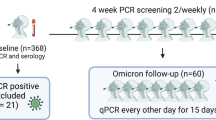
A summary of the study design and methodology is illustrated in Fig. 1.
Study design for the recruitment of participants and analysis of humoral and cellular responses to SARS-CoV-2. Asymptomatic young adults were identified by the asymptomatic testing service across 3 universities in the UK, utilising antibody and PCR tests. Asymptomatic participants were then recruited into the study with age and sex-matched participants. Samples were obtained before and after vaccination, then Wuhan and Omicron-specific assays were conducted. Created using BioRender
Results
Donor Characteristics
The demographics of participants is shown in Table 3. Samples were grouped according to infection status (negative, asymptomatic or symptomatic infection) and were taken from the same donors at two time points (one prior to vaccination with an infection date pre-August 2021 (unvaccinated) and one following two vaccinations (vaccinated)). All asymptomatic and symptomatic participants were infected with the Wuhan or Alpha variant. There were no significant differences in age, ethnicity, days since vaccination, vaccine type, or days between visits, between groups (p > 0.05) (statistical information can be found in supplementary Table 1).
Wuhan and Omicron Specific-Antibody Profiles
Despite trends of higher IgG titres in symptomatic individuals and lowest IgG titres in uninfected participants, there were no significant differences in anti-Wuhan spike IgG antibody responses between the three unvaccinated groups (Fig. 2Ai). Subsequent vaccination resulted in the median anti-Wuhan spike IgG antibody response significantly increasing in negative (p = 0.016) and symptomatic participants (p = 0.002), but not asymptomatic. Comparisons between the groups identified symptomatic participants had significantly higher Wuhan IgG compared to asymptomatic participants, post-vaccination (p = 0.039). Anti-Omicron spike IgG increased post-vaccination in negative participants (p = 0.039), but not asymptomatic or symptomatic (Fig. 2Aii). As shown for Wuhan-specific IgG, the median anti-Omicron spike IgG antibody response was also greater in symptomatic participants compared to asymptomatic participants, post-vaccination (p = 0.025).
Antibody response to SARS-CoV-2 spike in negative, asymptomatic and symptomatic participants. Wuhan and Omicron-specific IgG and IgA antibody titres were measured by ELISA and a neutralization assay was performed to quantify neutralization titres. Anti-spike IgG antibody responses for Wuhan (Ai) and Omicron (Aii) peptides. Wuhan (Bi) and Omicron (Bii) neutralization antibody responses. The correlation between anti-spike and neutralising antibodies for Wuhan (Ci) and Omicron (Cii). Salivary Wuhan-specific IgA antibody responses (D). Total N=102 (9 Negative Unvaccinated, 9 Negative Vaccinated, 23 Asymptomatic Unvaccinated, 23 Asymptomatic Vaccinated, 19 Symptomatic Unvaccinated, 19 Symptomatic Vaccinated). For Wuhan IgA, total N=68. Light blue bars = negative participants, dark blue bars = asymptomatic participants, green bars = symptomatic participants. *p<0.05, **p<0.01
Importantly, the pseudotyped Wuhan antibody neutralization assay showed symptomatic participants had significantly increased neutralization antibodies pre-vaccination (p = 0.010) as well as post-vaccination (p = 0.026), compared to asymptomatic participants (Fig. 2Bi). In contrast to Wuhan-specific neutralization, there were no differences between pre-vaccinated asymptomatic and symptomatic participants’ Omicron-specific neutralization (p>0.05), but there were still differences between post-vaccinated asymptomatic and symptomatic neutralization (p = 0.017) (Fig. 2Bii). Furthermore, neutralization correlated with total anti-spike antibody titre (r = 0.623, p < 0.0001) (Fig. 2Ci). Also as seen in the Wuhan specific responses, Omicron neutralization was correlated to Omicron-specific antibody titre (r = 0.394, p = 0.001) (Fig. 2Cii). Wuhan-specific salivary IgA was also analyzed but did not differ between groups pre- or post-vaccination (Fig. 2D). Anti-nucleocapsid antibody titres were also measured and showed no significant differences between pre- and post-vaccination or between groups (Supplementary Figure 3).
CD4+ T cell Responses to Wuhan and Omicron
CD4+ T cell activation markers and cytokine production were measured by flow cytometry after PBMC stimulation with either Wuhan or Omicron-specific peptide pools of the Spike protein. Pre-vaccination, the AIM assay demonstrated there were significantly fewer activated Wuhan spike-specific CD4+ T cells in asymptomatic participants compared to symptomatic participants (p = 0.029), as well as a trend of fewer activated spike-specific CD4+ T cells in negative participants (Fig. 3Ai). Notably, post-vaccination, there were no longer significant differences between symptomatic and asymptomatic activated Wuhan spike-specific CD4+ T cells. Vaccination also increased the percentage of activated CD4+ T cells across all groups, but only significantly in asymptomatic participants responding to Wuhan spike (p = 0.008). In Omicron spike-specific responses, CD4+ T cell activation was also low pre-vaccination, but across all groups (Fig. 3Aii). Similar to Wuhan, vaccination increased the percentage of activated Omicron spike-specific CD4+ T cells across all groups, but only significantly in asymptomatic participants (p = 0.020).
CD4+ T cell Responses to SARS-CoV-2 Spike Peptides in Negative, Asymptomatic and Symptomatic Adults. The percentage of activated spike-specific CD4+ T cells in response to Wuhan (Ai) and Omicron (Aii) peptides. The total percentage of cytokine-positive CD4+ T cells in response to Wuhan (Bi) and Omicron (Bii) spike peptides. Median percent of Wuhan spike-specific cytokine-positive T cells (Ci) and Omicron spike-specific cytokine-positive T cells (Cii). Individual graphs for CD4+ T cells producing IFNγ-only are shown to highlight significant differences between groups in response to Wuhan spike (Di) and Omicron spike (Dii). Total N=102 (9 Negative Unvaccinated, 9 Negative Vaccinated, 23 Asymptomatic Unvaccinated, 23 Asymptomatic Vaccinated, 19 Symptomatic Unvaccinated, 19 Symptomatic Vaccinated). Light blue bars = negative participants, dark blue bars = asymptomatic participants, green bars = symptomatic participants
Pre-vaccination, the ICS assay found no significant differences in the percentage of cytokine-producing CD4+ T cells between groups, in response to Wuhan or Omicron spike proteins (Fig. 3Bi-Bii). Post-vaccination, the percentage of Wuhan spike-specific CD4+ T cells producing cytokines in the negative group significantly increased (p = 0.019), but not in participants with previous infection. Similarly, Omicron spike-specific cytokine-positive CD4+ T cells significantly increased post-vaccination (p = 0.027), but also in the asymptomatic group (p = 0.039). Surprisingly, negative participants who had been vaccinated had a significantly higher percentage of cytokine-producing T cells, specific to Omicron spike, compared to symptomatic participants (p = 0.015).
The heatmap breakdown of CD4+ T cells producing different combinations of cytokines (Fig. 3Ci-Cii) indicates trends of increased IFN-γ-only producing CD4+ T cells after vaccination in negative and asymptomatic participants, in response to Wuhan and Omicron spike proteins. Statistical analysis found this was significantly increased post-vaccination in negative participants only, in response to Wuhan (p = 0.016) and Omicron (p = 0.039) (Fig. 3Dii-Diii). In response to Omicron spike, this IFN-γ only subset was also significantly higher in the negative vaccinated compared to symptomatic vaccinated participants (p = 0.047).
Finally, we observed no correlations between IgG responses and CD4+ T cell responses (Data can be found in Supplementary Figure 4i and iii).
CD8+ T cell Responses to Wuhan and Omicron
CD8+ T cell cytokine production was measured in parallel by flow cytometry after stimulation with the same Wuhan and Omicron spike-specific peptides. As shown in CD4+ T cells, the AIM assay demonstrated negative and asymptomatic participants had fewer activated Wuhan spike-specific CD8+ T cells compared to symptomatic participants, pre-vaccination. Statistical analyzes reinforced the finding that asymptomatic participants had significantly fewer Wuhan spike-specific CD8+ T cells than symptomatic participants (p = 0.021). Post-vaccination, there were no significant increases in Wuhan spike-specific activation of CD8+ T cells (Fig. 4Ai), but surprisingly, the CD8+ T cells did significantly increase after vaccination in response to Omicron spike in asymptomatic participants only (p = 0.004) (Fig. 4Aii).
CD8+ T cell Responses to SARS-CoV-2 Spike Peptides in Negative, Asymptomatic and Symptomatic Adults. The percentage of activated spike-specific CD8+ T cells in response to Wuhan (Ai) and Omicron (Aii) peptides. The percentage of cytokine-positive CD8+ T cells in response to Wuhan (Bi) and Omicron (Bii) spike peptides. Cytokine combinations expressed by Wuhan (Ci) and Omicron (Cii) spike-specific CD8+ T cells. Total N=102 (9 Negative Unvaccinated, 9 Negative Vaccinated, 23 Asymptomatic Unvaccinated, 23 Asymptomatic Vaccinated, 19 Symptomatic Unvaccinated, 19 Symptomatic Vaccinated). Light blue bars = negative participants, dark blue bars = asymptomatic participants, green bars = symptomatic participants
Pre-vaccination, negative participants had significantly fewer Wuhan spike-specific CD8+ cytokine-positive T cells, compared to asymptomatic (p = 0.035) and symptomatic (p = 0.023) participants (Fig 4. Bi). Vaccination significantly increased the percentage of Wuhan spike-specific CD8+ T cells producing cytokines in negative participants (p = 0.019). In contrast, Omicron spike stimulation resulted in no significant differences pre- or post- vaccination or between groups (Fig 4. Bii).
Analysis of the different cytokines expressed by Wuhan and Omicron spike-specific CD8+ T cells (Fig. 4Ci-Cii) showed similar patterns to CD4+ T cell cytokine production, where the median percentage of IFN-γ-only producing CD8+ T cells was increased in negative vaccinated participants. However, none of these differences between groups reached statistical significance (p > 0.05).
Finally, we observed no correlations between IgG responses and CD8+ T cell responses (Data can be found in Supplementary Figure 4ii and iv).
Multi-dimensional Analysis of Wuhan-specific T cells
Multi-dimensional analysis was performed as an unbiased analysis of Wuhan-specific, cytokine-producing T cells, to investigate whether there were any differences which were not identified by conventional flow analysis. The multidimensional clustering analysis, using FlowSOM, grouped the Wuhan-stimulated PBMCs into a total of 28 clusters with different expression markers (Fig. 5a-b). To determine if there were any differences between cytokine-positive T cell phenotypes of subjects following Wuhan spike peptide exposure, the percentage of cells present from each subject group in each cluster was measured (Fig. 5c). Statistical analysis found two clusters (Fig. 5d) were significantly different between groups, and their tSNE plots are presented (Fig. 5e).
Wuhan-stimulated T cell Clustering Analysis. (a) FlowSOM clusters from Wuhan spike-exposed T cells presented as a tSNE plot. (b) The number of events in each FlowSOM cluster. (c) % of cluster occupied by negative, asymptomatic, and symptomatic subject groups before and after vaccination. (d) Statistical analysis of the differences between clusters 1 and 2, between groups. (e) tSNE plots of cluster 1 and 2 to identify location and size of cluster. (f) T cell marker expression of clusters 1 and 2. *=p<0.05, **=p<0.01, N=19. Light blue bars = negative participants, dark blue bars = asymptomatic participants, green bars = symptomatic participants
A two-way ANOVA indicated the cell population ‘Cluster 1’ was significantly higher in asymptomatic vaccinated than asymptomatic unvaccinated and symptomatic unvaccinated adults (p<0.05). Despite trends of symptomatic vaccinated increased compared to unvaccinated, this was not statistically significant. To identify the phenotype of the cluster, Cluster Explorer analysis was run (Fig. 5f), and the results indicate cluster 1 was CD4+ effector memory (CCR7-/CD45RA+) cells which expressed CD27, CD28, CD95, CD69, and produced IFN-γ and TNFα (but not IL-2).
In addition, the cell population ‘Cluster 2’ was significantly higher in symptomatic participants compared to asymptomatic participants, pre-vaccination (p<0.01), but post-vaccination there were no significant differences. Cluster 2 was identified as CD4+ T cells with a naïve phenotype (CD454RA+/CCR7+/CD27+/CD28+), expressing CD152, CD38, and producing IL-2 only.
Multi-dimensional Clustering Analysis of Omicron-specific T cells
Clustering analysis of Omicron spike-specific, cytokine-producing T cells identified 27 meta-clusters with different expression markers (Fig. 6a-b). The percentage of cells present from each participant group in each cluster was measured (Fig. 6c), and a two-way ANOVA found one cluster (Fig. 6d) was significantly different between groups, of which the cluster tSNE plot is presented (Fig. 6e). The results indicated the cells in Cluster 22 were significantly higher in asymptomatic (p = 0.0448) and symptomatic (p = 0.042) participants post-vaccination, compared to pre-vaccination. Cluster Explorer analysis helped identify the phenotype of cluster 22 (Fig. 6f), which was CD4+ effector memory T cells (CD45RA-/CCR7+) producing high amounts of IFNγ and low TNFα, as well as the expression of CD28, CD27 and CD95, notably similar to Cluster 1 from Wuhan clustering analysis.
Omicron-stimulated T cell Clustering Analysis. (a) FlowSOM clusters from Omicron spike-exposed T cells presented as a tSNE plot. (b) The number of events in each FlowSOM cluster. (c) % of cluster occupied by negative, asymptomatic, and symptomatic subject groups before and after vaccination. (d) Statistical analysis of the differences between cluster 22 between groups. (e) tSNE plots of cluster 22 to identify location and size of cluster. (f) T cell marker expression of cluster 22. *=p<0.05, N=19
A combined multi-dimensional analysis of both Wuhan and Omicron T cells was performed to decipher whether peptide-responsive cells form the same clusters. The clustering analysis (found in Supplementary Figure 5) shows 33 clusters, and a two-way ANOVA found no significant differences between Wuhan and Omicron for each cluster (p>0.05).
Discussion
To investigate long-term immunity to SARS-CoV-2, measuring T cell responses in addition to antibody responses is crucial, as T cells underpin long-lasting immunity. Despite existing studies investigating the immune response in asymptomatic disease [14, 15, 20,21,22], asymptomatic infection is less well-characterised, especially in a largely young and healthy demographic, and often in the absence of a true asymptomatic diagnosis. Thus, this well-defined, cohort study recruited young asymptomatic adults, identified by an established asymptomatic testing service across 3 UK centres, and age/sex-matched symptomatic and uninfected controls. We show deep-phenotyping of T cell responses, in addition to analysing antibody titres, together demonstrate asymptomatic young adults exhibit decreased humoral and cellular responses to natural SARS-CoV-2 infection, compared to those with symptomatic infection.
Traditionally, systemically administered vaccines are thought to generate strong IgG responses, which provide strong protection against lower respiratory tract disease [23, 24]. In agreement with this previous research, levels of IgG specific to Wuhan spike proteins increased after vaccination in all subject groups, but interestingly, not significantly in asymptomatic participants. This lack of significance may be due to the large variation in antibodies detected prior to vaccination in this group. Weaker IgG responses in vaccinated asymptomatic individuals were further reinforced by significantly lower Wuhan and Omicron-specific IgG titres, compared to those of vaccinated symptomatic participants. Despite participant samples being obtained before the Omicron variant emerged, Omicron-specific IgG also significantly increased after vaccination, but only in the uninfected (negative) group, indicating some ability of the vaccine to protect against future SARS-CoV-2 strains. Existing literature found primary vaccination with current COVID-19 vaccines and previous SARS-CoV-2 infections offered low protection against Omicron BA.1 and BA.2 infection [25]. Along with IgG playing an important role in humoral immunity, IgA has also been shown to have an important early role in the neutralization of SARS-CoV-2 virus after infection [26]; here, we show the presence of IgA after infection in all groups, but vaccination did not increase IgA titres. In fact, although not statistically significant, IgA titres slightly decreased after vaccination. This is consistent with previous literature which found salivary IgA does not increase after 2 doses of the mRNA SARS-CoV-2 vaccine [27]. Despite lowest SARS-CoV-2 specific antibody levels in uninfected individuals, the lack of significant differences between uninfected and asymptomatic or symptomatic participants are likely due to the presence of existing antibodies from previous seasonal coronavirus exposure, indicating a cross-reactivity with SARS-CoV-2 peptides [28,29,30].
In addition to weaker IgG responses, asymptomatic participants also exhibited fewer Wuhan-specific neutralization antibodies pre- and post-vaccination, as well as fewer Omicron-specific neutralization antibodies post-vaccination, which is also demonstrated in previous research [15, 31,32,33]. There is a protective effect of neutralization antibodies against future re-infection [34], suggesting symptomatic individuals could be better protected against SARS-CoV-2 re-infection compared to asymptomatic individuals. As expected, both Wuhan and Omicron neutralising antibodies positively correlated to total anti-spike antibody titre. Overall, the antibody response results are congruent with existing literature [35,36,37], which states symptomatic individuals produce a greater magnitude of antibody response, compared to asymptomatic individuals. Thus, despite this being a young and healthy cohort, asymptomatic infection still resulted in a weaker humoral response to SARS-CoV-2, before and after vaccination, implying symptomatic cases could be more likely to provide increased protective immune responses following infection and vaccination. In addition, asymptomatic individuals have reduced humoral protection against future strains, as the responses to Omicron were lower than symptomatic IgG and neutralization responses.
Compared to antibodies, T cells mediate a faster and more potent response to SARS-CoV-2 [11], thus, cognate T cell immune responses before/after vaccination were also analyzed. Asymptomatic individuals have been shown to produce an efficient memory T cell response to SARS-CoV-2 during the convalescent phase [14, 20]. However, conventional flow cytometric analysis of Wuhan spike-stimulated T cells indicated unvaccinated asymptomatic participants had significantly fewer responding CD4+ and CD8+ T cells, similar to that of the uninfected young adults. This highlights asymptomatic infection results in a reduced cellular response to SARS-CoV-2, which could be explained by a more effective immune response during initial exposure, resulting in this less robust response after exposure. Although these differences dissipated after vaccination, resulting in no significant differences between groups post-vaccination. Thus, vaccination is key in providing a more robust cellular response in asymptomatic individuals. In agreement with Sekine et al. [20], T cell cytokine analysis indicated CD4+ and CD8+ T cells producing IFN-γ only was the dominant response in both asymptomatic and symptomatic adults. Surprisingly, the percentage of activated and cytokine-producing CD4+ T cells was highest in negative vaccinated individuals, in response to Wuhan and Omicron. These observations were likely due to CD4+ T cells producing IFN-γ only, which were significantly increased post-vaccination in negative participants in response to Wuhan and Omicron. This indicates vaccination gives a differential immune response compared to natural infection.
As previously mentioned, the participants were infected prior to the Omicron outbreak. Thus, it is unsurprising that Omicron-specific activation of CD4+ and CD8+ T cells were reduced compared to Wuhan-specific stimulation. These reduced responses predict a lack of cellular immunity to future variants of SARS-CoV-2, despite previous infection or vaccination. These results are supported by a study of Wuhan-infected health-care workers who were subsequently triple-vaccinated, showing a lower magnitude of T cell responses to the Omicron spike peptide than individuals who had not been initially infected with Wuhan [38]. These results suggest immunological imprinting through exposure to the ancestral strain of SARS-CoV-2 impairs the cross-reactivity of the response to emerging variants of SARS-CoV-2. In addition, Omicron variants can bind in a separate register to the HLA-II heterodimer and abrogate T cell responses, which could further explain these results [39]. In contrast, we show a combined multi-clustering analysis of T cells responding to both Wuhan and Omicron found no significant differences in phenotype between Wuhan and Omicron-responsive T cells. In addition, prior research indicates no differences in T cell responses to Wuhan or Omicron, suggesting T cells generated in response to vaccination or previous SARS-CoV-2 infection can cross-recognize Omicron [40].
Multi-dimensional clustering analysis pinpointed additional populations that differed between groups, highlighting the importance of unbiased multi-dimensional analysis. Omicron-specific CD4+ effector memory T cells producing high amount of IFN-γ and low TNFα were significantly higher in symptomatic vaccinated individuals compared to symptomatic unvaccinated individuals. A similar cluster was identified after exposure to Wuhan, but higher in asymptomatic vaccinated compared to unvaccinated individuals. This is as expected as this suggests this group of CD4+ effector memory T cells increases after vaccination, which is representative of a long-lived immune response, characteristic after vaccination [11]. Notably, a population of CD4+/CD45RA+/CCR7+ T cells were also identified by clustering analysis as significantly different among groups, where symptomatic unvaccinated adults had a significantly higher percentage of activated CD4+/CD45RA+/CCR7+ T cells producing IL-2, compared to asymptomatic unvaccinated adults. This population may be naïve CD4+ T cells, which are crucial in SARS-CoV-2 peptide recognition by antigen presenting cells, and produce IL-2 to differentiate into memory cells [41]. Thus, an increased population in symptomatic adults indicates a higher potential to generate CD4+ memory cells, and subsequently a longer-lasting immune memory. Furthermore, research suggests this population may in fact be revertants; CD4+ memory T cells reverting back to a ‘naïve’ phenotype upon activation [42,43,44], as unlike conventional naïve T cells, this CD4+ subset are activated and producing IL-2.
In conclusion, utilising this well-defined young adult cohort, we identify key differences in both SARS-CoV-2 humoral and cellular immunity, dependent on the presence of symptoms during infection. We demonstrate asymptomatic young adults present decreased antibody and T cell responses to Wuhan and Omicron, pre-vaccination. Post-vaccination, antibody responses are still inferior, but T cell immunity resolves to levels seen in the symptomatic group, highlighting the requirement of targeted vaccines to improve antibody and T cell responses following asymptomatic SARS-CoV-2 infection.
Data Availability
All data required to evaluate the conclusions in the paper are present in the manuscript or its appendix. Further information on the study protocol or de-identified datasets generated and analyzed within this publication are available from the corresponding author on reasonable request.
References
Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–36.
Mohapatra RK, Pintilie L, Kandi V, Sarangi AK, Das D, Sahu R, et al. The recent challenges of highly contagious COVID-19, causing respiratory infections: symptoms, diagnosis, transmission, possible vaccines, animal models, and immunotherapy. Chem Biol Drug Des. 2020;96(5):1187–208.
Sah P, Fitzpatrick MC, Zimmer CF, Abdollahi E, Juden-Kelly L, Moghadas SM, Singer BH, Galvani AP. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118(34):e2109229118. https://doi.org/10.1073/pnas.2109229118.
Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17(9):e1003346.
Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–6.
Tsitsilonis OE, Paraskevis D, Lianidou E, Terpos E, Akalestos A, Pierros V, Kostaki EG, Kastritis E, Moutsatsou P, Politou M, Scorilas A, Sphicopoulos T, Thomaidis N, Trougakos IP, Tsakris A, Voulgaris N, Daskalaki CC, Evangelakou Z, Fouki C, Gianniou DD, … Sfikakis PP. SARS-CoV-2 Infection is asymptomatic in nearly half of adults with robust anti-spike protein receptor-binding domain antibody response. Vaccines. 2021;9(3):207. https://doi.org/10.3390/vaccines9030207
Wang B, Andraweera P, Elliott S, Mohammed H, Lassi Z, Twigger A, et al. Asymptomatic SARS-CoV-2 infection by age: a global systematic review and meta-analysis. Pediatr Infect Dis J. 2023;42(3):232–9.
Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–8.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11.
Muecksch F, Wise H, Templeton K, Batchelor B, Squires M, McCance K, et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: a serological analysis. The Lancet Microbe. 2022;3(7):e493–502.
Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–93.
Scurr MJ, Lippiatt G, Capitani L, Bentley K, Lauder SN, Smart K, et al. Magnitude of venous or capillary blood-derived SARS-CoV-2-specific T cell response determines COVID-19 immunity. Nat Commun. 2022;13(1):5422.
Jarjour NN, Masopust D, Jameson SC. T cell memory: understanding COVID-19. Immunity. 2021;54(1):14–8.
Le Bert N, Clapham HE, Tan AT, Chia WN, Tham CYL, Lim JM, Kunasegaran K, Tan LWL, Dutertre CA, Shankar N, Lim JME, Sun LJ, Zahari M, Tun ZM, Kumar V, Lim BL, Lim SH, Chia A, Tan YJ, Tambyah PA, … Tam CC. Highly unctional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5):e20202617. https://doi.org/10.1084/jem.20202617.
Chan YH, Fong SW, Poh CM, Carissimo G, Yeo NK, Amrun SN, et al. Asymptomatic COVID-19: disease tolerance with efficient anti-viral immunity against SARS-CoV-2. EMBO Mol Med. 2021;13(6):e14045.
Xie C, Li Q, Li L, Peng X, Ling Z, Xiao B, et al. Association of early inflammation with age and asymptomatic disease in COVID-19. J Inflamm Res. 2021;14:1207–16.
Ferreira I, Lee CYC, Foster WS, Abdullahi A, Dratva LM, Tuong ZK, et al. Atypical B cells and impaired SARS-CoV-2 neutralization following heterologous vaccination in the elderly. Cell Rep. 2023;42(8):112991.
van der Maaten L, Hinton G. Viualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–605.
Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A. 2015;87(7):636–45.
Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–68.e14.
Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med. 2021;27(5):904–16.
Soares-Schanoski A, Sauerwald N, Goforth CW, Periasamy S, Weir DL, Lizewski S, et al. Asymptomatic SARS-CoV-2 infection is associated with higher levels of serum IL-17C, matrix metalloproteinase 10 and fibroblast growth factors than mild symptomatic COVID-19. Front Immunol. 2022;13:821730.
Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccin Immunother. 2016;12(4):1070–9.
Wei J, Pouwels KB, Stoesser N, Matthews PC, Diamond I, Studley R, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28(5):1072–82.
Andeweg SP, de Gier B, Eggink D, van den Ende C, van Maarseveen N, Ali L, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13(1):4738.
Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, … Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. https://doi.org/10.1126/scitranslmed.abd2223.
Sheikh-Mohamed S, Isho B, Chao GYC, Zuo M, Cohen C, Lustig Y, et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15(5):799–808.
Hicks J, Klumpp-Thomas C, Kalish H, Shunmugavel A, Mehalko J, Denson JP, et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. J Clin Immunol. 2021;41(5):906–13.
Nguyen TTN, Choo EM, Nakamura Y, Suzuki R, Shiina T, Shin-I T, et al. Pre-existing cross-reactive neutralizing activity against SARS-CoV-2 and seasonal coronaviruses prior to the COVID-19 pandemic (2014-2019) with limited immunity against recent emerging SARS-CoV-2 variants, Vietnam. Int J Infect Dis. 2024;139:109–17.
Song G, He W-T, Callaghan S, Anzanello F, Huang D, Ricketts J, et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat Commun. 2021;12(1):2938.
Jeewandara C, Jayathilaka D, Gomes L, Wijewickrama A, Narangoda E, Idampitiya D, et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021;11(1):2062.
Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–42.
Sancilio A, Schrock JM, Demonbreun AR, D'Aquila RT, Mustanski B, Vaught LA, et al. COVID-19 symptom severity predicts neutralizing antibody activity in a community-based serological study. Sci Rep. 2022;12(1):12269.
Chen X, Xu Y, Xie Y, Song W, Hu Y, Yisimayi A, Yang S, Shao F, Geng L, Wang Y, Gao H, Shi Y, Zhang S, Jin R, Shen Z, Cao Y. Protective effect of plasma neutralization from prior SARS-CoV-2 Omicron infection against BA.5 subvariant symptomatic reinfection. The Lancet regional health. Western Pacific. 2023;33:100758. https://doi.org/10.1016/j.lanwpc.2023.100758.
Choe PG, Kang CK, Suh HJ, Jung J, Song KH, Bang JH, et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(1):327–9.
Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–4.
Holder KA, Ings DP, Harnum DOA, Russell RS, Grant MD. Moderate to severe SARS-CoV-2 infection primes vaccine-induced immunity more effectively than asymptomatic or mild infection. npj Vaccines. 2022;7(1):122.
Reynolds CJ, Pade C, Gibbons JM, Otter AD, Lin KM, Muñoz Sandoval D, et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377(6603):eabq1841.
Chen Y, Mason GH, Scourfield DO, Greenshields-Watson A, Haigh TA, Sewell AK, et al. Structural definition of HLA class II-presented SARS-CoV-2 epitopes reveals a mechanism to escape pre-existing CD4(+) T cell immunity. Cell Rep. 2023;42(8):112827.
Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–92.
Heller KN, Gurer C, Münz C. Virus-specific CD4+ T cells: ready for direct attack. J Exp Med. 2006;203(4):805–8.
Hargreaves M, Bell EB. Identical expression of CD45R isoforms by CD45RC+ ‘revertant’ memory and CD45RC+ naive CD4 T cells. Immunology. 1997;91(3):323–30.
Piatosa B, Wolska-Kuśnierz B, Tkaczyk K, Heropolitanska-Pliszka E, Grycuk U, Wakulinska A, et al. T lymphocytes in patients with nijmegen breakage syndrome demonstrate features of exhaustion and senescence in flow cytometric evaluation of maturation pathway. Front Immunol. 2020;11:1319.
Frumento G, Verma K, Croft W, White A, Zuo J, Nagy Z, et al. Homeostatic cytokines drive epigenetic reprogramming of activated T cells into a “Naive-Memory” phenotype. iScience. 2020;23(4):100989.
Acknowledgements
Nottingham Acknowledgements:
We thank the University of Nottingham Asymptomatic Testing service for identifying volunteers for the study, as well as the volunteers for their participation in this study.
We would like to thank Cripps Health Centre, Nottingham for obtaining blood samples and supporting the research.
We would also like to thank Professor Dame Jessica Corner for her involvement in the original grant application that funded this research, as well as Professor Philip Quinlan and Dr Maria Arruda.
Cambridge Acknowledgements:
We thank NIHR BioResource volunteers for their participation, and gratefully acknowledge NIHR BioResource centres, NHS Trusts and staff for their contribution. We thank the National Institute for Health and Care Research, NHS Blood and Transplant, and Health Data Research UK as part of the Digital Innovation Hub Programme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
We would like to thank Research Nurses Stewart Fuller, Marina Metaxaki and Mahin Bagheri and all our participants from the University of Cambridge Asymptomatic Screening Programme.
We would also like to thank Professor Patrick Maxwell for his involvement in the original grant application that funded this research.
Cardiff Acknowledgements:
We thank the team at Cardiff University who established the Asymptomatic Testing service, as well as the volunteers for their participation in this study.
We would also like to thank Professor Andrew D. Westwell for his involvement in the original grant application that funded this research.
Funding
This work was supported by two UKRI COVID- 19 National Core Study Immunity programmes (Coordinating COVID-19 asymptomatic testing programmes in university settings: providing insight on acquired immunity across the student population and Asymptomatic COVID-19 in Education (ACE) Immunity Study) to LF, MW and AGo. The ID7000C spectral cell analyzer was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) to LF and DO (Grant Ref BB/T017619/1). Ago was also funded by a wellcome Trust Collaborator Award Grant (209213/Z/17/Z). ImmunoServ were supported by an Innovate UK/Small Business Research Initiative award (10007867) and a UKRI COVID- 19 National Core Study Immunity programmes (SARS-CoV-2 Optimal Cellular Assays).
Author information
Authors and Affiliations
Contributions
GH, NG, DT, LB, GS, and MB recruited patients and processed blood for PBMC and sera isolation, and processed saliva samples. MPe, MPo, MM, BK, and MW processed blood for PBMC and sera isolation and processed saliva samples. SL, MS, LC, SB, TR, KS, MS, AGa, and MM all performed venipuncture and processed blood for PBMC and sera isolation, and ran T cell/serology assays to determine SARS-CoV-2 positivity. BK, MPe, and MM performed neutralization assays. BK and MW designed the omicron peptide pools. EB, DO, MW and LF designed and optimised the cellular assays. GH, NG, and RS performed cellular assays. TH-C prepared all reagents. GH, DO and LF conducted cellular assay analysis. HJ and PT performed antibody titre assays and analysis. GH wrote the manuscript with comments from LF, MW, AGo, BK, MPe, and MPo. LF, MW, and AGo designed the study, acquired the funding, and were site-specific project coordinators, and LF managed the project.
Corresponding author
Ethics declarations
Ethics Approval
Ethics approval for the study was granted by the University of Nottingham Medical School Ethics Committee (FMHS 96-0920), Cambridge COVID-19 NIHR BioResource joint Consent Form (Research Ethics Committee (NRES REC Ref 17/EE/0025), and Cardiff University School of Medical Research Ethics Committee (SMREC 21/01).
Consent to Participate
All participants provided written informed consent.
Conflict of Interest
MJS is a founder of and holds equity in ImmunoServ Ltd. AG is a founder of ImmunoServ Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3170 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hopkins, G., Gomez, N., Tucis, D. et al. Lower Humoral and Cellular Immunity Following Asymptomatic SARS-CoV-2 Infection Compared to Symptomatic Infection in Education (The ACE Cohort). J Clin Immunol 44, 147 (2024). https://doi.org/10.1007/s10875-024-01739-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10875-024-01739-0