Abstract
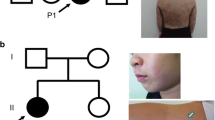
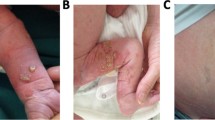
While most missense mutations of the IKBKG gene typically result in Ectodermal Dysplasia with Immunodeficiency, there have been rare reported instances of missense mutations of the IKBKG gene causing both Incontinentia Pigmenti (IP) and immunodeficiency in female patients. In this study, we described an atypical IP case in a 19-year-old girl, characterized by hyperpigmented and verrucous skin areas over the entire body. Remarkably, she experienced recurrent red papules whenever she had a feverish upper respiratory tract infection. Immunohistochemical staining unveiled a substantial accumulation of CD68+ macrophages alongside the TNF-α positive cells in the dermis tissue of new pustules, with increased apoptotic basal keratinocytes in the epidermis tissue of these lesions. Starting from the age of 8 years old, the patient suffered from severe and sustained chronic respiratory mucous membrane scar hyperplasia and occluded subglottic lumen. In addition to elevated erythrocyte sedimentation rate values, inflammatory cells were observed in the pathologic lesions of endobronchial biopsies and Bronchoalveolar Lavage Fluid (BALF) smear. Further histological analysis revealed a destructive bronchus epithelium integrity with extensive necrosis. Simultaneously, the patient experienced recurrent incomplete intestinal obstructions and lips contracture. The patient’s BALF sample displayed an augmented profile of proinflammatory cytokines and chemokines, suggesting a potential link to systemic hyperinflammation, possibly underlying the pathogenic injuries affecting the subglottic, respiratory, and digestive systems. Furthermore, the patient presented with recurrent pneumonias and multiple warts accompanied by a T+BlowNKlow immunophenotype. Next generation sequencing showed that the patient carried a novel de novo germline heterozygous missense mutation in the IKBKG gene (c. 821T>C, p. L274P), located in the highly conserved CC2 domain. TA-cloning sequencing of patient’s cDNA yielded 30 mutant transcripts out of 44 clones. In silico analysis indicated that the hydrogen bond present between Ala270 and Leu274 in the wild-type NEMO was disrupted by the Leu274Pro mutation. However, this mutation did not affect NEMO expression in peripheral blood mononuclear cells (PBMCs). Moreover, patient PBMCs exhibited significantly impaired TNF-α production following Lipopolysaccharide (LPS) stimulation. X-chromosome inactivation in T cells and neutrophils were not severely skewed. Reduced levels of IκBα phosphorylation and degradation in patient's PBMCs were observed. The NF-κB luciferase reporter assay conducted using IKBKG-deficient HEK293T cells revealed a significant reduction in NF-kB activity upon LPS stimulation. These findings adds to the ever-growing knowledge on female IP that might contribute to the better understanding of this challenging disorder.






Similar content being viewed by others
Data Availability
Not applicable.
References
Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733.
Maubach G, Schmädicke AC, Naumann M. NEMO links nuclear factor-kappaB to human diseases. Trends Mol Med. 2017;23:1138–55.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651.
Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2005;203:21–37.
Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–43.
Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Curr Opin Immunol. 2004;16:34–41.
Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177. e16.
Fusco F, Pescatore A, Conte MI, Mirabelli P, Paciolla M, Esposito E, et al. EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-kappaB pathway can cause immunodeficiency and/or inflammation. Int Rev Immunol. 2015;34:445–59.
Cammarata-Scalisi F, Fusco F, Ursini MV. Incontinentia pigmenti. Actas Dermosifiliogr (Engl Ed). 2019;110:273–8.
Minić S, Trpinac D, Obradović M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85:536–42.
Martinez-Gayosso A, Garcia-Romero MT. Incontinentia pigmenti: multisistemic genodermatosis. Bol Med Hosp Infant Mex. 2020;77(3):112–8
Fusco F, Paciolla M, Conte MI, Pescatore A, Esposito E, Mirabelli P, et al. Incontinentia pigmenti: report on data from 2000 to 2013. Orphanet J Rare Dis. 2014;9:93.
Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000;107:343–9.
Boisson B, Honda Y, Ajiro M, Bustamante J, Bendavid M, Gennery AR, et al. Rescue of recurrent deep intronic mutation underlying cell type-dependent quantitative NEMO deficiency. J Clin Invest. 2019;129(2):583–97.
Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405(6785):466–72.
Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am J Hum Genet. 2000;67:1555–62.
Fusco F, Pescatore A, Bal E, Ghoul A, Paciolla M, Lioi MB, et al. Alterations of the IKBKG locus and diseases: an update and a report of 13 novel mutations. Hum Mutat. 2008;29:595–604.
Dupuis-Girod S, Corradini N, Hadj-Rabia S, Fournet JC, Faivre L, Le Deist F, et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002;109:e97.
Ohnishi H, Kishimoto Y, Taguchi T, Kawamoto N, Nakama M, Kawai T, et al. Immunodeficiency in two female patients with incontinentia pigmenti with heterozygous NEMO mutation diagnosed by LPS unresponsiveness. J Clin Immunol. 2017;37:529–38.
Kosaki K, Shimasaki N, Fukushima H, Hara M, Ogata T, Matsuo N. Female patient showing hypohidrotic ectodermal dysplasia and immunodeficiency (HED-ID). Am J Hum Genet. 2001;69:664–6.
Martinez-Pomar N, Munoz-Saa I, Heine-Suner D, Martin A, Smahi A, Matamoros N. A new mutation in exon 7 of NEMO gene: late skewed X-chromosome inactivation in an incontinentia pigmenti female patient with immunodeficiency. Hum Genet. 2005;118:458–65.
Mou W, Yang S, Guo R, Fu L, Zhang L, Guo W, et al. A novel homozygous TTC7A missense mutation results in familial multiple intestinal atresia and combined immunodeficiency. Front Immunol. 2021;12:759308.
Kremserova S, Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol. 2020;2087:33–42.
Mou W, Gao L, He J, Yin J, Xu B, Gui J. Compound heterozygous DCLRE1C mutations lead to clinically typical severe combined immunodeficiency presenting with graft versus host disease. Immunogenetics. 2021;73:425–34.
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–39.
Nenci A, Huth M, Funteh A, Schmidt-Supprian M, Bloch W, Metzger D, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum Mol Genet. 2006;15:531–42.
Liao SL, Lai SH, Huang JL, Lee NC, Lee WI. Serial cytokine expressions in infants with incontinentia pigmenti. Immunobiology. 2013;218:772–9.
Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61.
Mitani Y, Wada T, Matsuda Y, Sakai S, Yachie A. XL-EDA-ID Presenting with congenital duodenal atresia and perforations. J Clin Immunol. 2018;38:733–5.
Márquez Balbás G, González-Enseñat MA, Vicente A, Creus-Vila L, Antón J, Umbert-Millet P. Incontinentia pigmenti and bipolar aphthosis: an unusual combination. ISRN Dermatol. 2011;2011:814186.
Cheng LE, Kanwar B, Tcheurekdjian H, Grenert JP, Muskat M, Heyman MB, et al. Persistent systemic inflammation and atypical enterocolitis in patients with NEMO syndrome. Clin Immunol. 2009;132:124–31.
Parrish JE, Scheuerle AE, Lewis RA, Levy ML, Nelson DL. Selection against mutant alleles in blood leukocytes is a consistent feature in incontinentia pigmenti type 2. Hum Mol Genet. 1996;5:1777–83.
Conte MI, Pescatore A, Paciolla M, Esposito E, Miano MG, Lioi MB, et al. Insight into IKBKG/NEMO locus: report of new mutations and complex genomic rearrangements leading to incontinentia pigmenti disease. Hum Mutat. 2014;35:165–77.
Fusco F, Bardaro T, Fimiani G, Mercadante V, Miano MG, Falco G, et al. Molecular analysis of the genetic defect in a large cohort of IP patients and identification of novel NEMO mutations interfering with NF-kappaB activation. Hum Mol Genet. 2004;13:1763–73.
Mizukami T, Obara M, Nishikomori R, Kawai T, Tahara Y, Sameshima N, et al. Successful treatment with infliximab for inflammatory colitis in a patient with X-linked anhidrotic ectodermal dysplasia with immunodeficiency. J Clin Immunol. 2012;32(1):39–49.
Klemann C, Pannicke U, Morris-Rosendahl DJ, Vlantis K, Rizzi M, Uhlig H, et al. Transplantation from a symptomatic carrier sister restores host defenses but does not prevent colitis in NEMO deficiency. Clin Immunol. 2016;164:52–6.
Miot C, Imai K, Imai C, Mancini AJ, Kucuk ZY, Kawai T, et al. Hematopoietic stem cell transplantation in 29 patients hemizygous for hypomorphic IKBKG/NEMO mutations. Blood. 2017;130(12):1456–67.
Code Availability
Not applicable.
Funding
This study was supported by grants from National Natural Science Foundation of China (No. 82173084, 82002129) and the fourth batch of public welfare and development from Beijing Municipal Health Commission (No. 2021-9).
Author information
Authors and Affiliations
Contributions
WM, JH, JG, and XL designed most of the studies. WM carried out much of the work together with JH, ZZ, LG, LF, JL, AJ, YP, TY, YG, LC, HW, JL, QQ, and BX. WM, JH, JG, and XL analyzed the data. WM drafted the manuscript and all authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Beijing Children’s Hospital.
Consent to Participate
Clinical information and blood samples were collected from the patient, the parents, and controls, all of whom had given their prior informed consent to participation in the study. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Consent for Publication
All authors consent to publish the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Supplementary Figure 1. Persistent microcytic hypochromic anemia. (A-B) Air-conduction (AC) and bone-conduction (BC) pure tone averages (PTA) were calculated based on four-point values at 500, 1000, 2000, and 3000 Hz in accordance with the American Academy of Otolaryngology-Head and Neck Surgery Hearing Committee minimal reporting standards. The AC-PTA and BC-PTA of left ear (A) was 120 and 67.5 dB, respectively while the AC-PTA and BC-PTA of right ear (B) was 115 and 67.5 dB, respectively. (C) Blood HGB value during therapy. (D) Blood MCHC value during therapy. (E) Blood MCV value during therapy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mou, W., Zhao, Z., Gao, L. et al. An Atypical Incontinentia Pigmenti Female with Persistent Mucocutaneous Hyperinflammation and Immunodeficiency Caused by a Novel Germline IKBKG Missense Mutation. J Clin Immunol 43, 2165–2180 (2023). https://doi.org/10.1007/s10875-023-01564-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-023-01564-x