Abstract
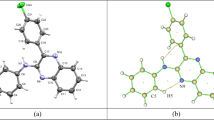
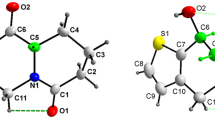
The structure of four spiro[chromeno[4,3-b]quinoline-6,1′-cycloalkan]-7-amine (1–4) was determined by X-ray diffraction methods. The quinoline rings were obtained as almost planar structures, with an RMS deviation within a range of 0.0345–0.0641 Å, while the chromene rings are not planar. Moreover, the cycloalkane rings are attached to the chromene in spiro form. Three of the four compounds crystallize as solvates—one with ethanol and the other two with water. The crystal packing for compounds 1–4 is discussed in terms of intermolecular interactions, and it is shown that the crystal packing of these heterocycles is governed by: strong O–H···N and N–H···O hydrogen bonds; weak C–H···O and C–H···N hydrogen bonds; and very weak C–H···Cl, C–H···Br, and C–H···π interactions. Also, π···π and lone pair···π interactions are present in the crystal packing of the heterocycles. These interactions lead to the formation of endless chains along a plane, as well as supramolecular dimers and the arrangement of molecules in layers. Additionally, UV–Vis absorption spectra properties are also discussed.
Graphical Abstract
The structure of four spiro[chromeno[4,3-b]quinoline-6,1’-cycloalkan]-7-amines are determined by single crystal X-ray diffraction. The crystal packing is discussed in terms of intermolecular interactions (strong O–H···N and N–H···O hydrogen bonds; weak C–H···O and C–H···N hydrogen bonds; and very weak C–H···Cl, C–H···Br, and C–H···π interactions). Also, π···π and lone pair π interactions are present in the crystal packing of the heterocycles.












Similar content being viewed by others
References
Romero A, Cacabelos R, Oset-Gasque MJ, Samadi A, Marco-Contelles J (2013) Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 23:1916–1922
Anand P, Singh B (2013) A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res 36:375–399
Hayour H, Bouraiou A, Bouacida S, Berree F, Carboni B, Roisnel T, Belfaitah A (2011) Synthesis and X-ray structures of new cycloalka[e]pyrano[2,3-b]pyridine derivatives: novel tacrine analogues. Tetrahedron Lett 52:4868–4871
Bonacorso HG, Silva LB, Rocha JBT, Nogara PA, Waczuk EP, Silva FD’A, Bueno DC, Kader YNAM., Martins MAP, Zanatta N (2015) Synthesis, biological evaluation and molecular docking study of 7-amine-spiro[chromeno[4,3-b]quinoline-6,1′-cycloalkanes] as new tacrine hybrids. Tetrahedron Lett 56:7024–7027
Martins MAP, Hörner M, Beck J, Tier AZ, Belladona AL, Meyer AR, Zanatta N, Bonacorso HG, Frizzo CP (2016) Polymorphism in an 18-membred macrocycle: an energetic and topological approach to undertand the supramolecular structure. CrystEngComm 18:3866–3876
Martins MAP, Meyer AR, Tier AZ, Longhi K, Ducati LC, Bonacorso HG, Zanatta N, Frizzo CP (2015) Proposal fro crystallization of 3-amino-4-halo-5-methylisoxazoles: an energetic and topological approach. CrystEngComm 17:7381–7391
Martins MAP, Frizzo CP, Martins ACL, Tier AZ, Grindi IM, Meyer AR, Bonacorso HG, Zanatta N (2014) Energetic and topological approach for characterization of supramolecular clusters in organic crystals. RSC Adv 4:44337–44349
Frizzo CP, Meyer AR, Caleffi GS, Rodrigues LV, Marzari MRB, Campos PT, Moreira DN, Bonacorso HG, Zanatta N, Martins MAP (2011) Influence of bulky and halogen substituents on crystal packing of pyrazolo[1,5-a]pyrimidines. J Mol Struct 1004:45–50
Campos PT, Machado P, Frizzo CP, Moreira DN, Meyer AR, Bonacorso HG, Zanatta N, Ducati L, Rittner R, Tormena CF, Martins MAP (2011) Structural invertigations of 5-hydroxy-4,5-dihydroisoxazoles. J Mol Struct 1006:462–468
Martins MAP, Moreira DN, Frizzo CP, Campos PT, Longhi K, Marzari MRB, Zanatta N, Bonacorso HG (2010) X-ray, semi-empirical MO calculations and π-electron delocalization of 1-cyanoacetyl-5-trifluoromethyl-5-hydroxy-4,5-dihydro-1H-pyrazoles. J Mol Struct 969:111–119
Machado P, Campos PT, Lima GR, Rosa FA, Flores AFC, Bonacorso HG, Zanatta N, Martins MAP (2009) Experimental and calculated structural parameters of 5-trihalomethyl-4,5-dihydro-1H-pyrazole derivatives, novel analgesic agents. J Mol Struct 917:176–182
Bruker (2006) APEX2 (Version 2.1), COSMO (Version 1.56), BIS (Version 2.0.1.9), SAINT (Version 7.3A) and SADABS (Version 2004/1) & XPREP (Version 2005/4), Bruker AXS Inc., Madison
Sheldrick GM (1997) SHELXS-97, program for crystal structure solution. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXL97, program for crystal structure refinement. University of Göttingen, Göttingen
Farrugia LJ (1997) ORTEP-III for Windows. J Appl Cryst 30:565
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) Tables of bond lengths determined by X-ray and neutron diffraction. part 1. bond lengths in organic compounds. J Chem Soc Perkin Trans II. https://doi.org/10.1039/P298700000S1
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76
Grabowski SJ (2004) Hydrogen bonding strength-measures based on geometric and topological parameters. J Phys Org Chem 17:18–31
Desiraju GR (2002) Hydrogen bridges in crystal engineering: interactions without borders. Acc Chem Res 35:565–573
The Cambridge Crystallographic Data Centre (CCDC) (2001–2016) Mercury, crystal structure visualization, CSD 3.9, (Build RC1)
Park YC, Lee JS (2006) Accurate ab initio binding energies of the benzene dimer. J Phys Chem A 110:5091–5095
Hill JG, Platts JA, Werner HJ (2006) Calculation of intermolecular interactions in the benzene dimer using coupled-cluster and local electron correlation methods. Phys Chem Chem Phys 8:4072–4078
Sinnokrot MO, Sherrill CD (2006) High-accuracy quantum mechanical studies of π - π interactions in benzene dimers. J Phys Chem A 110:10656–10668
Hunter CA, Sanders JKM (1990) The nature of.pi.-.pi. interactions. J Am Chem Soc 112:5525–5534
Pyykkö P (1997) Strong closed-shell interactions in inorganic chemistry. Chem Rev 97:597–636
Mooibroek TJ, Gamez P, Reedijk J (2008) Lone pair–π interactions: a new supramolecular bond? CrystEngComm 10:1501–1515
Acknowledgements
The authors thank the Coordination for Improvement of Higher Education Personnel (CAPES) for the fellowships and the National Council for Scientific and Technological Development (CNPq) for the financial support (Process Number 306.883/2015-5).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonacorso, H.G., Campos, P.T., Silva, L.B. et al. Structural Investigation, UV–Vis Analysis and Crystal Packing of Spiro[chromeno[4,3-b]quinoline-6,1′-cycloalkan]-7-amine: Novel Tacrine Hybrids by Single Crystal X-Ray Diffraction. J Chem Crystallogr 48, 19–31 (2018). https://doi.org/10.1007/s10870-018-0706-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0706-6