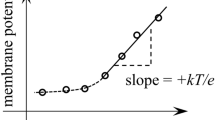
Abstract
In his Transmembrane Electrostatically Localized Proton hypothesis (TELP), James W. Lee has modeled the bioenergetic membrane as a simple capacitor. According to this model, the surface concentration of protons is completely independent of proton concentration in the bulk phase, and is linearly proportional to the transmembrane potential. Such a proportionality runs counter to the results of experimental measurements, molecular dynamics simulations, and electrostatics calculations. We show that the TELP model dramatically overestimates the surface concentration of protons, and we discuss the electrostatic reasons why a simple capacitor is not an appropriate model for the bioenergetic membrane.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Change history
19 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10863-022-09936-5
Notes
Some authors define ΔμH+ for proton export; in this case, Eq. 2 would be: pmf = +ΔμH+/zF.
Coulombs are abbreviated “coul” instead of the SI-recommended “C”, in order to distinguish it from the parameter “C” that represents capacitance.
Because a capacitor features equal and opposite charges on the two apposed plates, modelling the bioenergetic membrane as a protonic capacitor requires equal surface concentrations of H+ on the ‘P’ side, and OH− on the ‘N’ side. To my knowledge, neither Lee nor anyone else has attempted to test the validity of this assumption that [H+]surf(P) = [OH−]surf(N).
Lee uses equilibrium constants calculated from results published in (Saeed and Lee 2018). Serious problems with these calculated values are delineated in the Supplementary Information.
Using a thinner surface layer thickness of 5 Å instead of 10 Å (Lee 2015) worsens this problem, doubling the surface concentration of H+.
These are biochemical standard state values, ∆μ°′. ∆μ values calculated for typical physiological conditions (concentrations << 1 M) are less spontaneous.(Silverstein 2014)
References
Akaishi T (2018) Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction. Neural Regen Res 13:779
Azzone G, Benz R, Bertl A et al (1993) Transmembrane measurements across bioenergetic membranes. Biochim Biophys Acta Bioenerg 1183:1–3. https://doi.org/10.1016/0005-2728(93)90002-W
Cherepanov DA, Feniouk BA, Junge W, Mulkidjanian AY (2003) Low dielectric permittivity of water at the membrane interface: effect on the energy coupling mechanism in biological membranes. Biophys J 85:1307–1316
Dilley RA (2004) On why thylakoids energize ATP formation using either delocalized or localized proton gradients–a ca 2+ mediated role in thylakoid stress responses. Photosynth Res 80:245–263
Drachev LA, Kaulen AD, Skulachev VP (1984) Correlation of photochemical cycle, H+ release and uptake, and electric events in bacteriorhodopsin. FEBS Lett 178:331–335
Ferguson SJ (1985) Fully delocalised chemiosmotic or localised proton flow pathways in energy coupling?: a scrutiny of experimental evidence. Biochim Biophys Acta - Rev Bioenerg 811:47–95
Gabriel B, Teissie J (1996) Proton long-range migration along protein monolayers and its consequences on membrane coupling. Proc Natl Acad Sci U S A 93:14521–14525
Gopta OA, Cherepanov DA, Junge W, Mulkidjanian AY (1999) Proton transfer from the bulk to the bound ubiquinone Q(B) of the reaction center in chromatophores of Rhodobacter sphaeroides: retarded conveyance by neutral water. Proc Natl Acad Sci U S A 96:13159–13164
Gutman M, Nachliel E (1995) The dynamics of proton exchange between bulk and surface groups. Biochim Biophys Acta Bioenerg 1231:123–138
Gutman M, Huppert D, Pines E, Nachliel E (1981) Probing the micelle/water interface by rapid laser-induced proton pulse. Biochim Biophys Acta Biomembr 642:15–26
Heberle J, Riesle J, Thiedemann G et al (1994) Proton migration along the membrane surface and retarded surface to bulk transfer. Nature 370:379
Hodgkin AL, Horowicz P (1959a) Movements of Na and K in single muscle fibres. J Physiol 145:405–432
Hodgkin AL, Horowicz P (1959b) The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol 148:127–160
Hodgkin AL, Huxley AF (1952a) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544
Hodgkin AL, Huxley AF (1952b) Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116:449–472
Hodgkin AL, Huxley AF (1952c) The components of membrane conductance in the giant axon of Loligo. J Physiol 116:473–496
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108:37–77
Hodgkin AL, Huxley AF, Katz B (1952) Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol 116:424–448
Iuchi S, Chen H, Paesani F, Voth GA (2009) Hydrated excess proton at water- hydrophobic interfaces. J Phys Chem B 113:4017–4030
Junge D (1992) Nerve and muscle excitation (pp. 37-49)
Kell DB (1979) On the functional proton current pathway of electron transport phosphorylation: an electrodic view. Biochim Biophys Acta - Rev Bioenerg 549:55–99
Köfinger J, Dellago C (2008) Biasing the center of charge in molecular dynamics simulations with empirical valence bond models: free energetics of an excess proton in a water droplet. J Phys Chem B 112:2349–2356
Kotlyar A, Borovok N, Kiryati S et al (1994) The dynamics of proton transfer at the C side of the mitochondrial membrane: picosecond and microsecond measurements. Biochemistry (NY) 33:873–879
Lee JW (2012) Proton-electrostatics hypothesis for localized proton coupling bioenergetics. Bioenergetics 1:1–8
Lee JW (2013) Membrane surface charges attracted protons are not relevant to proton motive force. Bioenergetics 2:e114
Lee J (2015) Proton-electrostatic localization: explaining the bioenergetic conundrum in alkalophilic bacteria. Bioenergetics 4:1–8
Lee JW (2019) Electrostatically localized proton bioenergetics: better understanding membrane potential. Heliyon 5:e01961
Lee JW (2020a) Isothermal environmental heat energy utilization by transmembrane electrostatically localized protons at the liquid–membrane interface. ACS omega 5:17385–17395
Lee JW (2020b) Protonic capacitor: elucidating the biological significance of mitochondrial cristae formation. Sci Rep 10:1–14
Lee JW (2020c) Protonic conductor: better understanding neural resting and action potential. J Neurophysiol 124:1029–1044
Lee JW (2021a) Energy renewal: isothermal utilization of environmental heat energy with asymmetric structures. Entropy 23:665
Lee JW (2021b) Mitochondrial energetics with transmembrane electrostatically localized protons: do we have a thermotrophic feature? Sci Rep 11:1–13
Lee H-S, Tuckerman ME (2009) Ab initio molecular dynamics studies of the liquid- vapor Interface of an HCl solution. J Phys Chem A 113:2144–2151
Martí Cabarrocas A, Pérez González JJ, Madrenas Boadas J (2018) Action potential propagation: ion current or intramembrane electric field? Gen Physiol Biophys 37:71–82
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 191:144–148
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Physiol Rev 41:445–502
Mulkidjanian A, Cherepanov D, Heberle J, Junge W (2005) Proton transfer dynamics at membrane/water interface and mechanism of biological energy conversion. Biochem Mosc 70:251–256
Mulkidjanian AY, Heberle J, Cherepanov DA (2006) Protons@ interfaces: implications for biological energy conversion. Biochim Biophys Acta Bioenerg 1757:913–930
Nachliel E, Gutman M (1984) Kinetic analysis of proton transfer between reactants adsorbed to the same micelle. Eur J Biochem 143:83–88
Nachliel E, Gutman M (1996) Quantitative evaluation of the dynamics of proton transfer from photoactivated bacteriorhodopsin to the bulk. FEBS Lett 393:221–225
Nilsson T, Lundin CR, Nordlund G et al (2016) Lipid-mediated protein-protein interactions modulate respiration-driven ATP synthesis. Sci Rep 6:24113
Rieger B, Junge W, Busch KB (2014) Lateral pH gradient between OXPHOS complex IV and F 0 F 1 ATP-synthase in folded mitochondrial membranes. Nat Commun 5:1–7
Saeed HA, Lee JW (2015) Experimental demonstration of localized excess protons at a WaterMembrane Interface. Bioenergetics 4:147
Saeed HA, Lee JW (2018) Experimental determination of proton-cation exchange equilibrium constants at water-membrane Interface fundamental to bioenergetics. Water 9:116–140
Shi H, Hu Y, Odermatt PD et al (2021) Precise regulation of the relative rates of surface area and volume synthesis in bacterial cells growing in dynamic environments. Nat Commun 12:1–13
Silverstein TP (2014) An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F1F0-ATP synthases. J Bioenerg Biomembr 46:229–241. https://doi.org/10.1007/s10863-014-9547-y
Silverstein TP (2021) The proton in biochemistry: impacts on bioenergetics, biophysical chemistry, and bioorganic chemistry. Front Mol Biosci 8:1033. https://doi.org/10.3389/fmolb.2021.764099
Sjöholm J, Bergstrand J, Nilsson T et al (2017) The lateral distance between a proton pump and ATP synthase determines the ATP-synthesis rate. Sci Rep 7:2926
Springer A, Hagen V, Cherepanov DA et al (2011) Protons migrate along interfacial water without significant contributions from jumps between ionizable groups on the membrane surface. Proc Natl Acad Sci U S A 108:14461–14466
Toth A, Meyrat A, Stoldt S et al (2020) Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc Natl Acad Sci U S A 117:2412–2421
Tse Y-LS, Chen C, Lindberg GE et al (2015) Propensity of hydrated excess protons and hydroxide anions for the air–water interface. J Am Chem Soc 137:12610–12616
Vácha R, Buch V, Milet A et al (2007) Autoionization at the surface of neat water: is the top layer pH neutral, basic, or acidic? Phys Chem Chem Phys 9:4736–4747
Weichselbaum E, Österbauer M, Knyazev DG et al (2017) Origin of proton affinity to membrane/water interfaces. Sci Rep 7:1–8
Wick CD, Kuo I-FW, Mundy CJ, Dang LX (2007) The effect of polarizability for understanding the molecular structure of aqueous interfaces. J Chem Theor Comput 3:2002–2010
Williams RJ (1961) Possible functions of chains of catalysts. J Theor Biol 1:1–17
Williams RJ (1978) The multifarious couplings of energy transduction. Biochim Biophys Acta 505:1–44
Wolf MG, Grubmüller H, Groenhof G (2014) Anomalous surface diffusion of protons on lipid membranes. Biophys J 107:76–87
Yaguzhinsky LS, Boguslavsky LI, Volkov AG, Rakhmaninova AB (1976) Synthesis of ATP coupled with action of membrane protonic pumps at the octane–water interface. Nature 259:494–496
Yamashita T, Voth GA (2010) Properties of hydrated excess protons near phospholipid bilayers. J Phys Chem B 114:592–603
Zhang C, Knyazev DG, Vereshaga YA et al (2012) Water at hydrophobic interfaces delays proton surface-to-bulk transfer and provides a pathway for lateral proton diffusion. Proc Natl Acad Sci U S A 109:9744–9749
Zheng Y, Shojaei-Baghini E, Wang C, Sun Y (2013) Microfluidic characterization of specific membrane capacitance and cytoplasm conductivity of single cells. Biosens Bioelectron 42:496–502
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to revisions in the text body and equations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silverstein, T.P. A critique of the capacitor-based “Transmembrane Electrostatically Localized Proton” hypothesis. J Bioenerg Biomembr 54, 59–65 (2022). https://doi.org/10.1007/s10863-022-09931-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-022-09931-w