Abstract
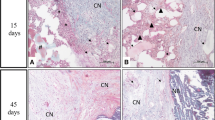
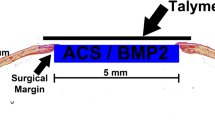
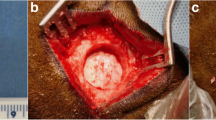
We have evaluated the capability of a collagen/poly glycolic acid (PGA) scaffold in regeneration of a calvarial bone defects in rabbits. 4 bone critical size defects (CSD) were created in the calvarial bone of each rabbit. The following 4 treatment modalities were tested (1) a collagen/PGA scaffold (0.52% w/w); (2) the collagen/PGA scaffold (0.52% w/w) seeded with adipose-derived mesenchymal stem cells (AD-MSCs, 1 × 106 cells per each defect); (3) AD-MSCs (1 × 106 cells) no scaffold material, and (4) blank control. The rabbits were then divided into 3 random groups (of 5) and the treatment outcomes were evaluated at 4, 8 and 12 weeks. New bone formation was histologically assessed. Experimental groups were analyzed by CT scan and real-time PCR. Histological analysis of bone defects treated with collagen/PGA alone exhibited significant fibrous connective tissue formation at the 12 weeks of treatments (P ≤ 0.05). There was no significant difference between collagen/PGA alone and collagen/PGA + AD-MSCs groups. The results were confirmed by CT scan data showing healing percentages of 34.20% for the collage/PGA group alone as compared to the control group and no difference with collagen/PGA containing AD-MSCs (1 × 106 cells). RT-PCR analysis also indicated no significant differences between collagen/PGA and collagen/PGA + AD-MSC groups, although both scaffold containing groups significantly express ALP and SIO rather than groups without scaffolds. Although there was no significant difference between the scaffolds containing cells with non-cellular scaffolds, our results indicated that the Collagen/PGA scaffold itself had a significant effect on wound healing as compared to the control group. Therefore, the collagen/PGA scaffold seems to be a promising candidate for research in bone regeneration.





Similar content being viewed by others
References
Shumrick KA, Smith CP. The use of cancellous bone for frontal sinus obliteration and reconstruction of frontal bony defects. Arch Otolaryngol Neck Surg. 1994;120:1003–9.
Aho AJ, Hirn M, Aro HT, Heikkila JT, Meurman O. Bone bank service in Finland: experience of bacteriologic, serologic and clinical results of the Turku Bone Bank 1972–1995. Acta Orthop Scand. 1998;69:559–65.
Sailer HF, Grätz KW, Kalavrezos ND. Frontal sinus fractures: principles of treatment and long term results after sinus obliteration with the use of lyophilized cartilage. J Cranio-Maxillofac Surg. 1998;26:235–42.
Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006;148:535–40.
Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–53.
Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–9.
Di Bella C, Farlie P, Penington AJ. Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Eng Part A. 2008;14:483–90.
Ahsan T, Nerem RM. Bioengineered tissues: the science, the technology, and the industry. Orthod Craniofac Res. 2005;8:134–40.
Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science (80-) Am Assoc Adv Sci. 1982;215:174–6.
Teramachi M, Nakamura T, Yamamoto Y, Kiyotani T, Takimoto Y, Shimizu Y. Porous-type tracheal prosthesis sealed with collagen sponge. Ann Thorac Surg. 1997;64:965–9.
Chamberlain LJ, Yannas IV, Hsu H, Strichartz GR, Spector M. Near‐terminus axonal structure and function following rat sciatic nerve regeneration through a collagen‐GAG matrix in a ten‐millimeter gap. J Neurosci Res. 2000;60:666–77.
Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annu Rev Biophys Bioeng. 1974;3:231–53.
Miyata T, Taira T, Noishiki Y. Collagen engineering for biomaterial use. Clin Mater. 1992;9:139–48.
Rault I, Frei V, Herbage D, Abdul-Malak N, Huc A. Evaluation of different chemical methods for cros-linking collagen gel, films and sponges. J Mater Sci Mater Med. 1996;7:215–21.
Friess W. Collagen–biomaterial for drug delivery1. Eur J Pharm Biopharm. 1998;45:113–36.
Rocha LB, Goissis G, Rossi MA. Biocompatibility of anionic collagen matrix as scaffold for bone healing. Biomaterials. 2002;23:449–56.
Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–86.
Mohajeri S, Hosseinkhani H, Golshan Ebrahimi N, Nikfarjam L, Soleimani M, Kajbafzadeh A-M. Proliferation and differentiation of mesenchymal stem cell on collagen sponge reinforced with polypropylene/polyethylene terephthalate blend fibers. Tissue Eng Part A. 2010;16:3821–30.
Toosi S, Naderi‐Meshkin H, Kalalinia F, Peivandi MT, HosseinKhani H, Bahrami AR, et al. PGA‐incorporated collagen: toward a biodegradable composite scaffold for bone‐tissue engineering. J Biomed Mater Res Part A. 2016;104:2020–8.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Yang M, Ma QJ, Dang GT, Ma KT, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7:273–81.
Halvorsen Y-DC, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–41.
Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP‐2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–9.
Hicok KC, Du Laney TV, Zhou YS, Halvorsen Y-DC, Hitt DC, Cooper LF, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–80.
Huang JI, Beanes SR, Zhu M, Lorenz HP, Hedrick MH, Benhaim P. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002;109:1033.
Toosi S, Naderi-Meshkin H, Kalalinia F, Peivandi MT, Hossein KH, Bahrami AR, et al. Comparative characteristics of mesenchymal stem cells derived from reamer-irrigator-aspirator, iliac crest bone marrow, and adipose tissue. Cell Mol Biol. 2016;62:8.
Lee JA, Parrett BM, Conejero JA, Laser J, Chen J, Kogon AJ, et al. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann Plast Surg. 2003;50:610–7.
Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60–8.
Moreira-Gonzalez A, Lobocki C, Barakat K, Andrus L, Bradford M, Gilsdorf M, et al. Evaluation of 45S5 bioactive glass combined as a bone substitute in the reconstruction of critical size calvarial defects in rabbits. J Craniofac Surg. 2005;16:63–70.
Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Cranio-Maxillofac Surg. 2004;32:370–3.
Edwards PC, Ruggiero S, Fantasia J, Burakoff R, Moorji SM, Paric E, et al. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005;12:75–86.
Dudas JR, Marra KG, Cooper GM, Penascino VM, Mooney MP, Jiang S, et al. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg. 2006;56:543–8.
Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture‐expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312.
Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, et al. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504.
Apel A, Groth A, Schlesinger S, Bruns H, Schemmer P, Büchler MW, et al. Suitability of human mesenchymal stem cells for gene therapy depends on the expansion medium. Exp Cell Res. 2009;315:498–507.
Toosi S, Naderi-Meshkin H, Kalalinia F, Peivandi MT, HosseinKhani H, Bahrami AR. et al. Comparative characteristics of mesenchymal stem cells derived from reamer-irrigator-aspirator, iliac crest bone marrow, and adipose tissue. Cell Mol Biol. 2016;36:68–74.
Lübke C, Ringe J, Krenn V, Fernahl G, Pelz S, Kreusch-Brinker R, et al. Growth characterization of neo porcine cartilage pellets and their use in an interactive culture model. Osteoarthr Cartil. 2005;13:478–87.
Naderi‐Meshkin H, Andreas K, Matin MM, Sittinger M, Bidkhori HR, Ahmadiankia N, et al. Chitosan‐based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int. 2014;38:72–84.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35.
Dell’Accio F, Vanlauwe J, Bellemans J, Neys J, De Bari C, Luyten FP. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21:123–31.
Chen G, Ushida T, Tateishi T. Fabrication of PLGA–collagen hybrid sponge. Chem Lett. 1999;1999:561–2.
Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229–34.
Cowan CM, Shi Y-Y, Aalami OO, Chou Y-F, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–7.
Li H, Lin G, Lue TF. Potential application of adipose tissue-derived stem cells for urological disease. Bladder. 2014;1:e2.
Hoseinzadeh HA, Asghari A, Abedi G, Akbarzadeh A, Sedaghat R. Effect of nano-capsules containing risedronate on calvarial bone formation in rabbit: radiography and biochemical investigation. Crescent J Med Biol Sci. 2018;5:29–33.
Mariano R, Messora M, de Morais A, Nagata M, Furlaneto F, Avelino C, et al. Bone healing in critical-size defects treated with platelet-rich plasma: a histologic and histometric study in the calvaria of diabetic rat. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;109:72–8.
Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175–85.
Bancroft GN, Mikos AG. Bone tissue engineering by cell transplantation. Tissue Eng Ther Use. 2001;5:151.
Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Regen Med II. 2005;94:1–22.
Läuchli S, Hafner J, Wehrmann C, French LE, Hunziker T. Post-surgical scalp wounds with exposed bone treated with a plant-derived wound therapeutic. J Wound Care. 2012;21:228–33.
Goldstein SA. Tissue engineering. Ann N Y Acad Sci. 2002;961:183–92.
Acknowledgements
The authors are indebted to the Research Council of Mashhad University of Medical Sciences, Iran, and National Institute for Medical Research Development (NIMAD), Tehran, Iran for approval and financial support of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toosi, S., Naderi-Meshkin, H., Kalalinia, F. et al. Bone defect healing is induced by collagen sponge/polyglycolic acid. J Mater Sci: Mater Med 30, 33 (2019). https://doi.org/10.1007/s10856-019-6235-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6235-9