Abstract
In the present study, a series of four different scaffolds were comparatively evaluated in a goat calvarial critical size defect model. Such studies are only rarely reported in the literature. In our work, E1001(1k), a member of a large combinational library of tyrosine-derived polycarbonates (TyrPC), was used to prepare two calcium phosphate hybrid, biodegradable bone scaffolds. In one formulation, the widely used β-tricalcium phosphate (β-TCP) was incorporated into the polymer scaffold. In the second formulation, a coating of dicalcium phosphate dihydrate (DCPD, also known as brushite) was used as the mineral phase. These scaffolds were evaluated for bone regeneration in goat calvarial 20-mm critical size defects (CSD) after 16 weeks. Results were compared with chronOS (a clinically used product) and E1001(1k)/β-TCP scaffolds, augmented with 400 μg of recombinant human bone morphogenetic protein-2 (rhBMP-2). Microcomputed tomography (micro-CT) and histomorphometry were used to assess bone regeneration within the defects. Histomorphometry showed that rhBMP-2-augmented E1001(1k)/β-TCP scaffolds completely healed the defect in all animals within 16 weeks. Among the hybrid scaffolds that were not augmented with rhBMP-2, the degree of bone regeneration within the defect area was low for the clinically used chronOS, which is a poly(lactide co-ε-caprolactone)/β-TCP hybrid scaffold. Similar results were obtained for E1001(1k)/β-TCP scaffolds, indicating that replacing poly(lactide co-ε-caprolactone) with E1001(1k) does not improve bone regeneration is this model. However, a statistically significant improvement of bone regeneration was observed for E1001(1k)/DCPD scaffolds. These scaffolds resulted in significant levels of bone regeneration in all animals and in complete bridging of the defect in three of six tests. This is the first report of a synthetic bone scaffold being able to heal a critical size calvarial defect in a large animal model without the addition of exogenous growth factors.
Lay Summary
Reconstruction of large bone defects is a significant clinical problem. The overwhelming majority of all research results are obtained in vitro or in small animal models (mouse, rat, rabbit) that cannot predict the clinical outcomes in humans. We address this problem by conducting our studies in a goat calvarial critical size defect model, which is widely regarded as predictive of human outcomes. Among the three rhBMP-2-free scaffolds tested, only one specific formulation, E1001(1k)/DCPD, resulted in massive bone ingrowth into the center of the defect in all animals and in complete bridging of the defect 50% of the animals. This is the first time, a synthetic bone scaffold was able to heal a critical size calvarial defect in a large animal model without the addition of biological growth factors. Given the high cost of biologically enhanced bone grafts and the regulatory complexities of their FDA market clearance, the development of E1001(1k)/DCPD hybrid scaffolds addresses a significant clinical need.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trauma, cancer, and congenital abnormalities can lead to critical size bone defects that are difficult to treat [1, 2]. The most successful treatment option is an autograft (vascularized bone from the patient) [1, 3]. However, the use of autologous bone has well-known limitations, such as donor site morbidity, increased surgery time, and limited availability [1, 4]. Due to these limitations, intense research efforts in academic laboratories and in industry are underway to develop synthetic regenerative bone scaffolds that can effectively treat large critical size bone defects.
The search for bone regenerative scaffolds that perform as well as autograft is not new. A vast amount of literature describes the performance of scaffolds based on in vitro and in vivo studies using a variety of ceramic materials, polymeric materials, and combinations of polymers with ceramics [5]. Most commonly, candidate bone scaffolds are tested in small animal models (mouse, rat, rabbit) and show variable degrees of bone regeneration. However, we found no prior reports of reproducible and robust regeneration of bone within large critical-size calvarial defects in the goat in the absence of exogenously added growth factors.
The most widely used growth factor is recombinant human BMP-2 (rhBMP-2). However, this is not an optimal solution. In a clinical setting, rhBMP-2 is only approved by the FDA for a limited number of indications, and side effects from off-label use have been reported [6].
Since calcium phosphates (CaPs) are generally considered osteoconductive, they have been widely investigated as composites, in combination with biodegradable polymers [1, 7]. The largest number of scientific publications report on hydroxyapatite (HA) and beta tri-calcium phosphate (β-TCP). These CaPs promote new bone growth through enhanced biocompatibility [8, 9], osteoconductivity, and osteointegration [8, 10]. In contrast, our group has focused on dicalcium phosphate dihydrate (DCDP or brushite), which is one of the precursors of biological apatite present in bone and tooth [11, 12]. Contrary to HA or β-TCP, DCPD is a metastable CaP phase at physiological conditions. Therefore, it has high solubility and resorbs faster than β-TCP and much faster than sintered HA [13, 14]. DCPD is widely used in hydraulic calcium phosphate cements and coatings for metallic implants, dating back to the 1990s [15, 16].
CaPs are commonly blended with polymer to produce composite scaffolds. chronOS and the E1001(1k)/β-TCP scaffolds were prepared in this fashion. However, this approach produces composite scaffolds in which the CaP phase is embedded within the polymer and only partially exposed at the surface. This reduces the rate of CaP dissolution and its ability to interact with cells that migrate into the scaffold. Consequently, only marginal bone regeneration is typically observed in large CSDs, and substantial amounts of osteogenic growth factors such as recombinant human bone morphogenetic protein-2 (rhBMP-2) are required to achieve significant bone regeneration within the scaffold. Recently, biomimetic coating methods based on immersion in simulated body fluid [17,18,19] or the alternate soaking process [20, 21] have been utilized to deposit a layer of calcium phosphate minerals on the surface of porous scaffolds [17, 22].
Our group has developed an E1001(1k) scaffold produced by porogen leaching combined with freeze-drying for bone regeneration [9, 23, 24]. E1001(1k) is a member of a large combinatorial library of tyrosine-derived polycarbonates (TyrPC) whose properties can be tailored for different tissue engineering applications by small variations in the polymer composition [24, 25]. E1001(1k) scaffolds have demonstrated favorable pore size, porosity, and interconnectivity for permitting in vivo bone and vascular ingrowth in rabbit calvarial defect models [9, 24, 26].
The objective of this study was to explore the bone regeneration potential of E1001(1k) scaffolds containing two different CaP mineral phases in the goat calvarial 20-mm CSD model in comparison with a clinically used product. Two types of E1001(1k)-based scaffolds were evaluated: (i) E1001(1k) scaffolds fabricated as a bulk composite with β-TCP (abbreviated as E1001(1k)/β-TCP scaffolds) and (ii) E1001(1k) scaffolds coated with DCPD (abbreviated as E1001(1k)/DCPD scaffolds). The bone regeneration potential of those scaffolds was compared with (i) chronOS, a commercially available bone graft substitute made of poly(lactide co-ε-caprolactone) and β-TCP, and (ii) E1001(1k)/β-TCP supplemented with 400 μg of rhBMP-2 per scaffold. To the best of our knowledge, this study is the first comparative report of several CaP/polymer hybrid scaffolds in a clinically predictive, large animal model.
Materials and Methods
Materials
Sodium chloride (NaCl), calcium chloride (CaCl2), and potassium phosphate dibasic trihydrate (K2HPO4·3H2O) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). The NaCl particles were sieved to select particles with sizes in the range of 212–425 μm and used as a porogen in scaffold fabrication. β-TCP (100 nm) powders were purchased from Berkeley Advanced Biomaterials, Inc. (Berkeley, CA). 1,4-Dioxane was obtained from Fischer Scientific (Pittsburgh, PA). Poly(DTE-co-10 mol% DT-co-1 mol% PEG1k carbonate), abbreviated E1001(1k), was synthesized according to published procedures [24] at the New Jersey Center for Biomaterials (Piscataway, NJ). DTE stands for desaminotyrosyl tyrosine ethyl ester, DT for desaminotyrosyl tyrosine, and PEG1k for poly(ethylene glycol) with a number average molecular weight of 1 kDa. chronOS was obtained from DePuy Synthes (West Chester, PA). rhBMP-2 was purchased from Wyeth-Genetics Institute, Inc. (Cambridge, MA).
Scaffold Fabrication
E1001(1k)/β-TCP scaffolds were prepared by a minor modification to our previously published salt leaching and freeze-drying method [9, 23]. Briefly, 12.2 g E1001(1k) polymer was dissolved in 8.54 ml deionized (DI) water and 52.46 ml 1,4-dioxane overnight. The polymer solution was uniformly mixed with 109.8 g NaCl and 12.2 g β-TCP powder using an overhead mixer (EuroStar Power Control Visc Stirrer, IKA Works, Inc., Wilmington, NC) at 500 rpm for 10 min. The mixture was then poured into two Teflon molds (60 mm in diameter × 20 mm in height). The samples were frozen at − 50 °C for 6 h and freeze-dried at 0 °C for 24 h and at 20 °C for another 24 h using a computer-controlled large-scale lyophilizer (LD85, Millrock Technology, Kinston, NY). After drying, disk-shaped scaffolds (20 mm in diameter) were punched out from the molds using a round, tubular puncher. The salt particles were completely removed by washing the scaffolds in distilled water. Then, the scaffolds were placed into a 6-mm-deep stainless steel holder. This facilitated the removal of the nonporous skins from the bottom and top of the scaffold with a razor blade, resulting in uniformly sized scaffolds with final dimensions of 20 mm diameter × 6 mm height. Finally, the scaffolds were dried in a lyophilizer at room temperature for 24 h.
E1001(1k)/DCPD scaffolds were prepared by coating E1001(1k) scaffolds with DCPD minerals using a modified alternate soaking process. Briefly, E1001(1k) scaffolds were first prepared using the salt leaching and freeze-drying process without β-TCP described above. Each E1001(1k) scaffold was placed into a separate well of a soaking chamber (16 wells per chamber) and immersed in 20 ml of 0.25 M CaCl2 solution (pH = 6). Vacuum (up to 30 inHg) was applied to the chamber for 1 min, followed by the rapid release to atmospheric pressure; this step was repeated five times to completely wet the scaffolds with CaCl2 solution. Then, the scaffolds were removed from the CaCl2 solution and pat-dried with a paper towel to remove excess solution on the scaffold surface. The scaffolds were then transferred to 0.25 M K2HPO4·3H2O solution (pH = 6), and the same vacuum and atmospheric pressure cycles were used to force the phosphate solution into the interior of the 3D scaffolds to react with the CaCl2 to form calcium phosphate. The scaffolds were alternated in calcium (Ca) and phosphate (P) solutions until 50% calcium phosphate by weight was obtained. The solutions were refreshed after every 5 cycles. Finally, scaffolds were dried in a lyophilizer for 24 h.
Scaffold Characterization
Samples of the freeze-dried scaffolds were cut using a razor blade and sputter coated with gold/palladium (30 mA, 2 min, SCD 004 sputter coater). The scaffold morphology and microstructure were observed using a scanning electron microscope (SEM, AMRAY-1830I) at an acceleration potential of 20 kV.
X-ray diffraction (XRD) patterns of scaffolds were obtained using a Philips X’Pert X-ray diffractometer operating at 40 kV and 40 mA (Cu-Kα radiation: λ = 1.5406). The scaffolds were scanned from 5° to 90° with a step size of 0.02° and scan step time of 1 s. The X-ray diffractogram was analyzed using PANalytical HighScore Plus software and compared with the standard library of known diffraction patterns, International Centre for Diffraction Data (ICDD), to identify the phase of the calcium phosphate present in the scaffolds. The percent of Ca and P (by weight) incorporated into the scaffolds was determined via elemental analysis using inductively coupled plasma optical emission spectrometry (ICP-OES, Intertek). The Ca-to-P ratio was calculated. Analysis was done in triplicate.
The porosity of the scaffolds was determined using a helium pycnometer (Porous Material, Inc). Brunauer-Emmett-Teller (BET) specific surface area of the scaffolds was determined using BET Sorptometer (Porous Material, Inc.). Scaffolds prior to implantation were also scanned in an eXplore Locus microcomputed tomography (micro-CT, GE Healthcare) at a voxel resolution of 20 μm, voltage of 80 kVp, and current of 150 mA. Three-dimensional images of the scaffolds were reconstructed from the scans by the micro-CT system software package. The total porosity, total pore volume, and largest pore volume were obtained.
The compressive elastic moduli of scaffolds were measured using ReNew MTS Systems 4 (crosshead speed of 0.5 mm/min and a load cell of 100 N). To more accurately mimic the mechanical properties of scaffolds in vivo, the scaffolds were pre-wet in phosphate-buffered saline (PBS) overnight and the mechanical tests were carried out in PBS at 37 °C. The data was collected and analyzed using TestWork 4 (Ver.4.11C; MTS Systems).
The molecular weight of the scaffolds was determined using gel permeation chromatography (GPC) relative to polystyrene standards following a published procedure [24].
Scaffold Sterilization
The scaffolds were sterilized using ethylene oxide (EtO) at Johnson & Johnson Sterility Assurance Group (Raritan, NJ). Scaffolds before and after sterilization were characterized according to the “Scaffold Characterization” section in triplicate.
Goat Calvarial Surgery and Necropsy
The Institutional Animal Care and Use Committee (IACUC) at Allegheny Singer Research Institute (Pittsburgh, PA) and the Department of Defense United States Army Medical Research and Materiel Command Animal Care and Use Review Office (ACURO) approved all surgical procedures involving animals. Proper handling, housing, care, and standard caprine food was given to the animals according to the guidelines posted by Allegheny Singer Research Institute’s IACUC, the Animal Welfare Act, and the National Research Council.
Adult skeletally mature Boer Cross female goats weighting 36–57 kg were used for the study. A randomized experiment was conducted to determine the effect of scaffold composition on healing in a critical sized calvarial defect (CSD, 20-mm diameter). Animals were anesthetized with an intravenous injection of propofol, and anesthetic maintenance was performed through inhalation of isoflurane gas. A 7-cm incision was made through the skin, and the periosteum was removed using Bovie electrocautery pen. A 20-mm calvarial defect (Fig. 1) was created along the sagittal suture just slightly posterior to the intersection of the coronal suture using a surgical trephine with copious physiological saline for irrigation. The dura was kept intact. One scaffold per craniotomy per goat was then randomly inserted (press fit). The treatment groups (Table 1) are (1) E1001(1k)/β-TCP scaffolds, (2) E1001(1k)/DCPD scaffolds, (3) E1001(1k)/β-TCP scaffolds + 400 μg rhBMP-2, and (4) chronOS. For scaffolds containing rhBMP-2, 200-μg rhBMP-2 in 325 μl of PBS was delivered dropwise via micropipette and dried in the biological safety cabinet (BSC) for 2 h. The scaffold was then turned over, and the second aliquot of 200-μg rhBMP-2 in 325 μl of PBS was applied on the other side of the scaffold. After drying for an additional 2 h in the BSC, the scaffold was ready for implantation. Due to the high porosity of the scaffold, the BMP solution rapidly infiltrated into the pores of the scaffold and penetrated the scaffold throughout its entire pore volume. Following insertion of the scaffold into the defect, deep tissue, including musculature, was closed with inverted resorbable sutures, and the skin was closed with a non-resorbable running/lock-stitch suture. Once the animals were upright and ambulatory, they were returned to the animal housing facility for the remainder of the study (16 weeks in life). At 16 weeks post-implantation, goats were euthanized humanely. The samples were explanted with the surrounding bone and grossly examined for signs of infection, inflammation, and bone resorption. The bone blocks were fixed in 10% neutral-buffered formalin and prepared for micro-CT and histological analyses.
Microcomputed Tomography
X-ray projections of the explants were collected using an eXplore Locus micro-CT (GE Healthcare) according to a published procedure (80 kVp; 500 mA, 30 min/sample, and 20 μm voxel resolution) [9]. Three-dimensional construction of the projection images was performed on a 4PC Unix Cluster (8 GB RAM, ~ 60 min to reconstruct each volume) using a modified tent-FDK cone beam algorithm. The images were segmented using predefined Hounsfield unit thresholds (calcium phosphate, cortical bone, trabecular/woven bone, and scaffold content > 3000, 2000–3000, 750–2000, and 300–750, respectively), and a 3D histomorphometric analysis was performed using MATLAB. The new bone volume (BV) and bone mineral density (BMD) were calculated. BV was normalized to total volume (TV) within the region of interest (ROI), as an indicator of the relative amount of newly formed bone.
Histology and Histomorphometry
The specimens were dehydrated in ascending concentration of ethanol from 50 to 100%, infiltrated with a clearing agent, then embedded in polymethylmethacrylate (PMMA). The PMMA blocks were mounted to Exakt plastic slides and cut off to render approximately 200-μm-thick sections with an Exakt 300 CP Band System, then ground to approximately 110 μm with an Exakt 400CS Grinding System, and finally polished to a 1-μm surface finish using a Buehler Handimet until reaching 100 ± 5 μm thickness. Plane sections cut at 45° from the sagittal suture through the center of the defects were stained with Stevenel’s Blue and counterstained with van Gieson’s picrofuchsin, which stains bone red and nonmineralized structures various shades of blue. Gigapixel images of the whole cross sections were obtained. An AOI was delineated, and the percentage area of new bone was calculated in relation to the total AOI. The linear distance of new bone ingrowth from the defect margins was measured and divided by the defect length to calculate linear ingrowth percentage (LI), an indicator of the spatial pattern of bone ingrowth.
Statistics
Statistical analyses were performed using single factor analysis of variance (ANOVA) followed by a multiple comparison post hoc test (Tukey-Kramer method) with a significance level established as p ≤ 0.05. All statistical analyses were carried out using GraphPad Prism 6 software package. All data were reported as mean ± standard deviation (SD).
Results
Scaffold Preparation and Characterization
The average dimensions of E1001(1k)-based scaffolds were 20.2 ± 0.1 mm (diameter) × 5.8 ± 0.1 mm (height), and the average mass was 359 ± 18 mg (n = 60). The microstructure of the scaffolds was analyzed using SEM (Fig. 2a). Both E1001(1k)-based scaffolds displayed a highly porous and interconnected structure with macropores ranging from 200 to 400 μm (top row of Fig. 2a). In addition, micropores with diameters less than 20 μm were observed within the walls of the macropores. Images at higher magnification (bottom row of Fig. 2a) show the detailed surface topography of the macropore walls. For E1001(1k)/β-TCP scaffolds, small particles were uniformly dispersed throughout the polymer matrix, and some particles were embedded within the matrix. For E1001(1k)/DCPD scaffolds, large plate-like crystals were exposed on the polymer surface and occupied some space within the macropores. In contrast, chronOS appeared relatively less porous, and the β-TCP particles appeared to be largely covered by the polymer.
a Representative SEM images of the macroporous networks (top row) and surface topologies (bottom row) of E1001(1k)/β-TCP scaffolds, E1001(1k)/DCPD scaffolds, and chronOS scaffolds at low and high magnifications, respectively. b X-ray diffractograms of E1001(1k)/β-TCP scaffolds, E1001(1k)/DCPD scaffolds, and chronOS
Figure 2b shows XRD scans of CaP-containing scaffolds. The scans of E1001(1k)/β-TCP scaffold and chronOS displayed characteristic peaks at 27.8°, 31.0°, and 34.4°, indicating the presence of β-TCP. Although chronOS contains a higher amount of β-TCP than E1001(1k)/β-TCP scaffolds (70 vs. 50 wt%, respectively), the characteristic peaks for β-TCP were at a lower intensity. This is likely due to the coverage of β-TCP particles by the polymer during the fabrication process of chronOS and is consistent with the SEM image (Fig. 2a). The XRD scans of E1001(1k)/DCPD scaffolds showed peaks at 11.7°, 20.9°, and 29.3°, which are characteristics of DCPD (ICDD #01-072-0713). The identity of the different calcium phosphates was further confirmed by the calcium-to-phosphate ratio measured from elemental analysis using inductively coupled plasma optical emission spectrometry. The Ca-to-P ratios of E1001(1k)/β-TCP scaffolds and chronOS were 1.57 ± 0.05 and 1.46 ± 0.02, respectively, which agrees with the theoretical value of 1.50 for β-TCP. The Ca-to-P ratio of E1001(1k)/DCPD scaffolds was 1.07 ± 0.01, corresponding to the expected theoretical value of 1.00 for DCPD.
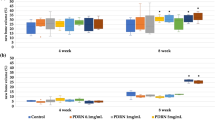
Next, we characterized several scaffold properties. We also examined whether our sterilization protocol influences scaffold properties by recording results before and after sterilization for the E1001(1k) scaffolds. Helium pyconometry measurements showed that the high overall porosity of E1001(1k)-based scaffolds was not diminished by the presence of the CaP minerals. Our results also showed that the E1001(1k)-based scaffolds were about 1.8 times more porous than chronOS (90% vs. 50%, respectively) (Fig. 3a). In addition, BET specific surface area (SSA) measurements showed that E1001(1k)-based scaffolds had a SSA of 0.22–0.35 m2/g, which was five times higher than that of chronOS (0.071 m2/g) (Fig. 3b). The high SSA of E1001(1k)-based scaffolds may be attributed to the large quantity of micropores present on the scaffolds’ pore walls, as shown in the SEM images of Fig. 2a. Moreover, E1001(1k)-based scaffolds demonstrated a slightly higher, although not statistically significant, compressive elastic modulus than chronOS (Fig. 3c). No significant effect of ethylene oxide (EtO) sterilization on scaffold porosity, BET SSA, compressive elastic modulus, and polymer molecular weight was measured (Fig. 3). In addition, no change in scaffold morphology and CaP phase was observed, suggesting that exposure to EtO gas is a suitable sterilization method for E1001(1k)-based scaffolds.
a Physical properties of E1001(1k)/β-TCP and E1001(1k)/DCPD scaffolds before and after ethylene oxide sterilization: a total scaffold porosity obtained from helium pyconometer. b BET specific surface area in m2/g. c Compressive elastic modulus in MPa. d Molecular weight of E1001(1k) in kDa. chronOS was purchased as sterilized 100 mm (long) × 6 mm (thick) rectangular strips. Disks (20 mm (diameter) × 6 mm (thick)) were punched out from the strips and characterized as a comparison. Data are reported as a mean ± standard deviation for n = 3 with a significance level of p < 0.05
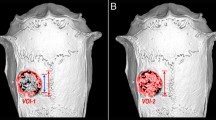
Micro-CT was used to generate 2D slices and 3D reconstructions of the scaffolds and showed that E1001(1k)-based scaffolds are highly porous with substantial interconnectivity (Fig. 4a, b). The micro-CT data were also used to calculate porosity (table inset in Fig. 4). Based on these calculations, E1001(1k) scaffolds and chronOS have porosities of ~ 40% and 30%, respectively, which was significantly lower than the values obtained using helium pyconometry. A possible explanation for the underestimation in porosity is that micro-CT has a limited voxel resolution (20 μm) which translates to an actual resolution of about 30 μm. Micro-CT could therefore not detect micropores less than about 20–30 μm. These pores were excluded from the reported overall porosity value. The micro-CT data was also used to determine the total pore volume and the largest pore volume. The largest pore volume was nearly equal to the total pore volume for all scaffolds, suggesting that all scaffolds had a very high degree of interconnectivity, where micro-CT recorded all of the interconnected pores as a single, very large pore (Fig. 4c). The largest pore volume was over 99% of the total pore volume for E1001(1k)-based scaffolds and chronOS, indicating that the majority of the pores were open. Blind or closed pores contributed less than 0.6% to the total pore volume.
Micro-CT analyses of scaffolds before implantation. a Representative three-dimensional reconstruction image of an E1001(1k)-based scaffold. b Cross-sectional image of an E1001(1k)-based scaffold. c An illustration of open, blind, and closed pores. d Summary table of the quantitative 3D morphometric analyses, including total scaffold porosity, total pore volume, largest pore volume, percent closed/blind pore, and percent open/interconnected pore.
Bone Regeneration in CSD Goat Calvaria
Ex Vivo Micro-CT Evaluation
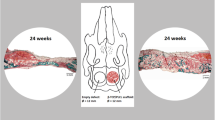
Micro-CT 2D coronal and transverse images of the calvarial specimens obtained 16 weeks post-treatment are shown in Fig. 5a. A qualitative comparison of the defects treated with scaffolds without rhBMP-2 suggests that E1001(1k)/DCPD scaffolds promoted the highest amount of new bone formation. It is noteworthy that the regenerated bone had grown into the center of the defect in all animals and had completely bridged the defect in three of six test animals. In contrast, defects treated with chronOS and E1001(1k)/β-TCP scaffolds contained newly formed bone only along the margins of the defect (at the bone-implant interface). Defects treated with E1001(1k)/β-TCP scaffolds supplemented with 400 μg rhBMP-2 had significantly more new bone formation as compared with all treatments without rhBMP-2. These defects were completely filled with newly regenerated bone, and bridging of the defects was observed in all test animals. Unfortunately, the quantitative evaluation of the micro-CT images was difficult, due to the need to distinguish between the CaPs left behind in the scaffolds at 16 weeks post implantation and the calcium content of newly regenerated bone. Specifically, the quantitative analyses of bone volume and bone mineral density presented in Fig. 5b is most probably affected by small changes in micro-CT gating. We addressed this inherent ambiguity by evaluating all defects also by histomorphometry.
Micro-CT analyses of scaffolds after explantation. a Representative 2D coronal (top) and transverse micro-CT images (bottom) of bone regeneration in the 20-mm goat calvarial defects treated with choronOS, E1001(1k)/ β-TCP, E1001(1k)/DCPD, and E1001(1k)/β-TCP supplemented with 400 μg of rhBMP-2 16 weeks post-implantation. b Quantitative analyses of the percentage of trabecular bone volume out of the total volume of interest (BV/TV%, left figure) and the bone mineral density (BMD, right figure) as indications of bone regeneration. Data are reported as the mean ± standard deviation for n = 6–7. The statistical analysis was done as described in the “Statistics” section. Asterisk (*) indicates a statistical significance difference with p < 0.05. Defects treated with E1001(1k)/β-TCP scaffolds supplemented with 400 μg rhBMP-2 have significantly higher BV/TV% values compared with those treated with E1001(1k)/β-TCP scaffolds (p = 0.0001), E1001(1k)/DCPD scaffolds (p = 0.0022), and chronOS (p < 0.0001); no significant difference was found between defects treated with E1001(1k)/β-TCP scaffolds, E1001(1k)/DCPD scaffolds, and chronOS. The BMD of defects treated with E1001(1k)/β-TCP scaffolds supplemented with 400 μg rhBMP-2 is significantly higher than that of defects treated with E1001(1k)/β-TCP scaffolds (p = 0.0008), E1001(1k)/DCPD scaffolds (p = 0.006), and chronOS (p = 0.0007); no significant difference was found between defects treated with E1001(1k)/β-TCP scaffolds, E1001(1k)/DCPD scaffolds, and chronOS
Ex Vivo Histology Evaluation
The histology sections were stained with Stevenel’s Blue and counterstained with van Gieson’s picrofuchsin (Fig. 6a). Normal cellular infiltration and bone healing with no signs of inflammatory response were observed in all defects regardless of the treatment group. Of the defects treated with scaffolds without rhBMP-2, substantial bone formation through the center of the defect was observed only in defects treated with E1001(1k)/DCPD scaffolds. In contrast, marginal bone formation restricted to the implant-native bone interface was noted in defects treated with E1001(1k)/β-TCP scaffolds and chronOS. Defects treated with E1001(1k)/β-TCP scaffolds + rhBMP-2 had the most robust trabecular bone formation. In addition, it appears that bone regeneration originated from the dura side and extended toward the superior side of the defect. Histological examination of the defects also shows that E1001(1k)/DCPD scaffolds were almost completely degraded, whereas substantial fragments of β-TCP were still visible (as black objects) in defects treated with chronOS and E1001(1k)/β-TCP scaffolds.
a Representative histological images of 20-mm goat calvarial defects treated with choronOS, E1001(1k)/ β-TCP, E1001(1k)/DCPD, and E1001(1k)/β-TCP supplemented with 400 μg of rhBMP-2 16 weeks post-implantation. Red arrows indicate the defect boundary. The un-decalcified sections were stained with Stevenel’s Blue and counterstained with van Gieson’s picrofuchsin , which stains bone red and non-mineralized structure various shades of blue. b Summary of 2D histomorphometric analyses. The percent of bone area is an indication of the amount of bone formation, and percent of linear trabecular bone ingrowth is an indication of the spatial pattern of bone ingrowth. Data are reported as the mean ± standard deviation for n = 6 or 7. The statistical analysis was done as described in the “Statistics” section. Asterisk (*) indicates a statistical significance difference with p < 0.05. Defects treated with E1001(1k)/DCPD scaffolds have a significantly higher % bone area compared with those treated with E1001(1k)/β-TCP scaffolds (p = 0.0066). There was no significant difference in % bone area in defects treated with either E1001(1k)/β-TCP scaffolds or chronOS. It is important to note that there is no significant difference in % bone area between defects treated with E1001(1k)/DCPD scaffolds and those treated with E1001(1k)/β-TCP scaffolds supplemented with 400 µg rhBMP-2. Defects treated with E1001(1k)/β-TCP scaffolds supplemented with 400 µg rhBMP-2 have significantly higher % bone area compared with those treated with E1001(1k)/β-TCP scaffolds (p = 0.0002) and chronOS (p = 0.0025). Defects treated with E1001(1k)/DCPD scaffolds have significantly higher linear trabecular bone ingrowth compared with those treated with E1001(1k)/β-TCP scaffolds (p < 0.0001) and chronOS (p < 0.0001). There was no significant difference in linear trabecular bone ingrowth in defects treated with either E1001(1k)/β-TCP scaffolds or chronOS. It is important to note that there is no significant difference in linear trabecular bone ingrowth between defects treated with E1001(1k)/DCPD scaffolds and those treated with E1001(1k)/β-TCP scaffolds supplemented with 400 µg rhBMP-2. Defects treated with E1001(1k)/β-TCP scaffolds supplemented with 400 µg rhBMP-2 have significantly higher % linear trabecular bone ingrowth compared with those treated with E1001(1k)/β-TCP scaffolds (p < 0.0001) and chronOS (p < 0.0001)
The percent new bone area (Fig. 6b) by treatment group was 23.7% for E1001(1k)/DCPD, 12.3% for E1001(1k)/β-TCP, 16.1% for chronOS, and 27.8% for E1001(1k)/β-TCP + rhBMP-2. Defects treated with E1001(1k)/DCPD scaffolds had statistically significantly more new bone area than those treated with E1001(1k)/β-TCP scaffolds or chronOS. Defects treated with E1001(1k)/β-TCP scaffolds that were augmented with 400 μg of rhBMP-2 had statistically significantly more bone area than E1001(1k)/β-TCP scaffolds or chronOS, but surprisingly, there was no statistically significant difference between E1001(1k)/DCPD scaffolds (no rhBMP-2) and E1001(1k)/β-TCP scaffolds that were augmented with 400 μg of rhBMP-2. Figure 6c shows that defects treated with E1001(1k)/DCPD scaffolds had significantly higher trabecular bone ingrowth relative to those treated with E1001(1k)/β-TCP and chronOS (85%, 20%, and 30%, respectively).
Discussion
In this study, we present a novel E1001(1k)/DCPD scaffold that may be the next-generation synthetic regenerative bone scaffold for the treatment of segmental bone defects. The E1001(1k)/DCPD treatment group achieved a superior osteogenic outcome in the goat calvarial CSD relative to chronOS, a commercial bone graft substitute, and the E1001(1k)/β-TCP group. An exceptional bridging of goat calvarial critical size defects was observed for E1001(1k)/DCPD scaffolds without the addition of exogenous biological growth factors such as rhBMP-2.
After conducting a series of bone regeneration studies in the rabbit calvarial CSD model [9, 24, 26], we selected the goat calvarial model as a clinically relevant large animal model. Goats have metabolic and bone remodeling rates similar to those of humans [27,28,29]. Goats also have a body weight comparable with humans and a body size suitable for the implantation of large human-sized implants [29]. Therefore, the results obtained from the calvarial CSD goat model can be used to predict the likely outcomes for the treatment of human patients. However, because of the high cost of using large animal models, these studies are rare. A recently published paper, using the goat calvarial CSD model for the evaluation of a bone regeneration scaffold [30], showed that poly(lactide-co-glycolide) (PLGA)/tricalcium phosphate (TCP) scaffolds prepared by low-temperature rapid prototyping failed to regenerate bone at 12 and 24 weeks without the addition of rhBMP-2. In addition, reviewing the existing bone regeneration studies using rats [31], rabbits [9, 26], goats [30, 32], sheep [33], and dogs [34, 35], no bone scaffold has demonstrated the ability to completely bridge a critical size defect without the addition of osteogenic growth factors. Most of these studies showed minimal bone formation limited to the margin of the defect, similar to the results we observed for defects treated with E1001(1k)/β-TCP and chronOS.
The exact mechanism leading to the superior performance of E1001(1k)/DCPD scaffolds is not yet known, but based on a significant body of prior literature, we discuss several possible contributing factors. Although we did not quantify the amount of residual calcium phosphate minerals at the 16-week time point, the histological slides presented in Fig. 6 indicate the presence of significant amounts of residual β-TCP (black areas) and the absence of DCPD residues. This is consistent with the known, high dissolution rate of DCPD [15, 16, 36, 37]. It has been suggested that high concentrations of Ca2+ ions released from CaP materials may mimic a bone resorption microenvironment, thus stimulating osteoblast proliferation and hMSCs differentiation by directly activating intracellular mechanisms through Ca-sensing receptors in osteoblastic cells, and encouraging bone mineralization and bone regeneration [38, 39]. For example, Barradas et al. reported that hMSCs cultured in vitro on β-TCP scaffolds expressed more osteogenic genes, including osteopontin (OP), osteocalcin (OC), bone sialoprotein (BSP), and BMP-2 than hMSCs cultured on HA scaffolds [40, 41]. Since these researchers designed the scaffolds to have the same 3D structure, they attributed their findings to the high solubility of β-TCP as compared with HA. A similar finding was observed in one of the prior in vitro study from our laboratory where hMSCs cultured on E1001(1k)/DCPD scaffolds expressed significant higher alkaline phosphatase (ALP) than hMSCs cultured on E1001(1k)/β-TCP scaffolds, likely due to the higher solubility of DCPD as compared with β-TCP [42].
Other contributing factors may be the polymer chemistry and pore structure. A series of studies in vitro and in the rabbit calvarial CSD model demonstrated that E1001(1k) has a high degree of bone biocompatibility and osteoconductivity [9, 23, 26]. These studies also described the bimodal pore structure of E1001(1k) scaffolds. High scaffold porosity is expected to allow migration and proliferation of osteoprogenitor cells as well as in vivo vascularization [36]. The pore size range of 200–400 μm (Fig. 2a) is within the optimal pore size distribution for bone regeneration scaffolds [36]. In addition to macroporosity, the walls of the macropores are perforated with a substantial amount of micropores (< 20 μm). Studies have reported that the presence of micropores and high surface area of scaffolds benefit angiogenesis and osteoconduction by enhancing endogenous protein adsorption, nutrition, and osteogenic factors transportation, as well as to ion exchange and bone-like apatite formation by dissolution and precipitation [36, 43, 44].
A third reason for the superior performance of E1001(1k)/DCPD scaffolds may be related to the method of scaffold preparation. Most CaP-containing scaffolds are prepared as composites using mechanical mixing techniques, where the CaPs are embedded as fillers within the polymer matrix. This method of fabrication generally results in the formation of a polymer skin covering the inorganic particles, which may limit the contact of migrating cells with the osteoconductive surfaces of the CaPs [17, 19, 45].
In our study, DCPD was precipitated onto pre-formed E1001(1k) scaffolds, which exposed all DCPD crystals at the surface and provided increased contact between the calcium phosphate mineral phase and cells as compared with E1001(1k)/β-TCP scaffolds and chronOS which were prepared as bulk composites. Unfortunately, it is not possible to deposit β-TCP onto the polymer surface, making it impossible to prepare β-TCP-containing scaffolds in the same way we prepared our DCPD-containing scaffolds. Therefore, a direct comparison between the effectiveness of DCPD and β-TCP as CaP additives cannot be made because each was incorporated into the E1001(1k) scaffold using a different fabrication process (coating vs. composite).
Finally, we note the relatively low dose of rhBMP-2 used in our study. Our previous work using the rabbit calvarial CSD model has shown that E1001(1k)/β-TCP scaffolds require a much lower dose of rhBMP-2 than used by other researchers [46]. Based on this observation, we selected 400 μg rhBMP-2 as the dose for the goat calvarial CSD. In contrast, Yu et al. used 1.0 mg with PLGA/TCP 3D scaffolds in the same goat CSD calvarial model [30], and Sheehan et al. used 1.4 mg per defect site delivered with a collagen sponge in Rhesus monkey calvarial defects [47].
Our study left a few questions unanswered. For example, additional studies are required to determine the lowest effective dose of rhBMP-2 required for E1001(1k)/DCPD scaffolds. Extending the study beyond 16 weeks would allow us to document the long-term outcomes of treating large defects with different E1001(1k)-based scaffolds. A future study could look at vascularization of the newly formed bone and bone turnover (remodeling) and quantify the mechanical properties of the regenerated bone and elaborate on the bone density in the regenerated defect as compared with the bone density of native calvaria.
Conclusions
In this study, we showed that E1001(1k)/DCPD scaffolds without rhBMP-2 augmentation are able to support significantly enhanced bone regeneration as compared with E1001(1k)/β-TCP scaffolds and the clinically used chronOS. These results are significant since they were obtained in a clinically predictive large animal model using critical size defects. We also showed that complete healing of critical size defects can be obtained by augmenting E1001(1k)/β-TCP scaffolds with a low dose of rhBMP-2. Histological images indicated that E1001(1k)/β-TCP scaffolds and chronOS regenerated bone only along the margins of the defect. In contrast, bridging across the defect was obtained with E1001(1k)/DCPD scaffolds. These results suggest that E1001(1k)/DCPD scaffolds are promising candidates for the development of next-generation synthetic bone void fillers for segmental bone defect repair.
References
De Long WG Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649–58. https://doi.org/10.2106/jbjs.F.00465.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408.
Gazdag A, Lane J, Glaser D, Forster R. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3(1):1–8.
Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res. 2010;132:15–30.
Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15–56.
Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008;2(2–3):81–96. https://doi.org/10.1002/term.74.
Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res B Appl Biomater. 2005;72(1):94–101. https://doi.org/10.1002/jbm.b.30136.
Cao H, Kuboyama N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46(2):386–95. https://doi.org/10.1016/j.bone.2009.09.031.
Kim J, Magno MH, Waters H, Doll BA, McBride S, Alvarez P, et al. Bone regeneration in a rabbit critical-sized calvarial model using tyrosine-derived polycarbonate scaffolds. Tissue Eng A. 2012;18(11–12):1132–9. https://doi.org/10.1089/ten.TEA.2011.0582.
LeGeros RZ. Preparation of octacalcium phosphate (OCP): a direct fast method. Calcif Tissue Int. 1985;37(2):194–7. https://doi.org/10.1007/bf02554841.
Johnsson MS, Nancollas GH. The role of brushite and octacalcium phosphate in apatite formation. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1992;3(1–2):61–82.
Kanzaki N, Onuma K, Treboux G, Ito A. Dissolution kinetics of dicalcium phosphate dihydrate under pseudophysiological conditions. J Cryst Growth. 2002;235(1–4):465–70. https://doi.org/10.1016/S0022-0248(01)01771-7.
LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395:81–98.
Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31(Supplement 4):D37–47. https://doi.org/10.1016/S0020-1383(00)80022-4.
Apelt D, Theiss F, El-Warrak AO, Zlinszky K, Bettschart-Wolfisberger R, Bohner M, et al. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials. 2004;25(7–8):1439–51.
Theiss F, Apelt D, Brand B, Kutter A, Zlinszky K, Bohner M, et al. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26(21):4383–94. https://doi.org/10.1016/j.biomaterials.2004.11.056.
Seyedjafari E, Soleimani M, Ghaemi N, Shabani I. Nanohydroxyapatite-coated electrospun poly(l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11(11):3118–25. https://doi.org/10.1021/bm1009238.
Nandakumar A, Yang L, Habibovic P, van Blitterswijk C. Calcium phosphate coated electrospun fiber matrices as scaffolds for bone tissue engineering. Langmuir. 2010;26(10):7380–7. https://doi.org/10.1021/la904406b.
Vaquette C, Ivanovski S, Hamlet SM, Hutmacher DW. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials. 2013;34(22):5538–51. https://doi.org/10.1016/j.biomaterials.2013.03.088.
Kim HJ, Kim U-J, Kim HS, Li C, Wada M, Leisk GG, et al. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42(6):1226–34. https://doi.org/10.1016/j.bone.2008.02.007.
Zhao J, Zhang Z, Wang S, Sun X, Zhang X, Chen J, et al. Apatite-coated silk fibroin scaffolds to healing mandibular border defects in canines. Bone. 2009;45(3):517–27. https://doi.org/10.1016/j.bone.2009.05.026.
Surmenev RA, Surmeneva MA, Ivanova AA. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—a review. Acta Biomaterialia. 2014;10(2):557–79. https://doi.org/10.1016/j.actbio.2013.10.036.
Kim J, Magno MH, Alvarez P, Darr A, Kohn J, Hollinger JO. Osteogenic differentiation of pre-osteoblasts on biomimetic tyrosine-derived polycarbonate scaffolds. Biomacromolecules. 2011;12(10):3520–7. https://doi.org/10.1021/bm200700d.
Magno MHR, Kim J, Srinivasan A, McBride S, Bolikal D, Darr A, et al. Synthesis, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds. J Mater Chem. 2010;20(40):8885–93. https://doi.org/10.1039/C0JM00868K.
Magno MHR. Optimization of tyrosine-derived polycarbonate terpolymers for bone regeneration scaffolds. 2012. https://doi.org/10.7282/T3GM86BN.
Kim J, Magno MH, Ortiz O, McBride S, Darr A, Kohn J, et al. Next-generation resorbable polymer scaffolds with surface-precipitated calcium phosphate coatings. Regenerative biomaterials. 2015;2(1):1–8. https://doi.org/10.1093/rb/rbu019.
Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schutz MA, Duda GN, et al. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30(12):2149–63. https://doi.org/10.1016/j.biomaterials.2008.12.050.
Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. European cells & materials. 2007;13:1–10.
Li Y, Chen S-K, Li L, Qin L, Wang X-L, Lai Y-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. Journal of Orthopaedic Translation. 2015;3(3):95–104. https://doi.org/10.1016/j.jot.2015.05.002.
Yu D, Li Q, Mu X, Chang T, Xiong Z. Bone regeneration of critical calvarial defect in goat model by PLGA/TCP/rhBMP-2 scaffolds prepared by low-temperature rapid-prototyping technology. Int J Oral Maxillofac Surg. 2008;37(10):929–34. https://doi.org/10.1016/j.ijom.2008.07.012.
Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13(3):619–27. https://doi.org/10.1089/ten.2006.0102.
Nienhuijs ME, Walboomers XF, Briest A, Merkx MA, Stoelinga PJ, Jansen JA. Healing of bone defects in the goat mandible, using COLLOSS E and beta-tricalciumphosphate. J Biomed Mater Res B Appl Biomater. 2010;92(2):517–24. https://doi.org/10.1002/jbm.b.31546.
Gugala Z, Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J Orthop Trauma. 1999;13(3):187–95.
Itoh T, Mochizuki M, Nishimura R, Matsunaga S, Kadosawa T, Kokubo S, et al. Repair of ulnar segmental defect by recombinant human bone morphogenetic protein-2 in dogs. J Vet Med Sci. 1998;60(4):451–8.
Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2000;18(2):289–302. https://doi.org/10.1002/jor.1100180218.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. https://doi.org/10.1016/j.biomaterials.2005.02.002.
Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 2006;1(3):317–32.
Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010;46(3):571–6. https://doi.org/10.1016/j.bone.2009.07.082.
Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–74. https://doi.org/10.1016/j.biomaterials.2011.01.004.
Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33(11):3205–15. https://doi.org/10.1016/j.biomaterials.2012.01.020.
Barradas AM, Monticone V, Hulsman M, Danoux C, Fernandes H, Tahmasebi Birgani Z, et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr Biol. 2013;5(7):920–31.
Chen SS. Process development, optimization and preclinical evaluation of calcium phosphate containing polymer scaffolds for bone regeneration. 2016. https://doi.org/10.7282/T3JM2CQ0.
Wei J, Jia J, Wu F, Wei S, Zhou H, Zhang H, et al. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31(6):1260–9. https://doi.org/10.1016/j.biomaterials.2009.11.005.
Lan Levengood SK, Polak SJ, Wheeler MB, Maki AJ, Clark SG, Jamison RD, et al. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials. 2010;31(13):3552–63. https://doi.org/10.1016/j.biomaterials.2010.01.052.
He C, Xiao G, Jin X, Sun C, Ma PX. Electrodeposition on nanofibrous polymer scaffolds: rapid mineralization, tunable calcium phosphate composition and topography. Adv Funct Mater. 2010;20(20):3568–76. https://doi.org/10.1002/adfm.201000993.
Kim J, McBride S, Donovan A, Darr A, Magno MH, Hollinger JO. Tyrosine-derived polycarbonate scaffolds for bone regeneration in a rabbit radius critical-size defect model. Biomed Mater. 2015;10(3):035001. https://doi.org/10.1088/1748-6041/10/3/035001.
Sheehan JP, Sheehan JM, Seeherman H, Quigg M, Helm GA. The safety and utility of recombinant human bone morphogenetic protein-2 for cranial procedures in a nonhuman primate model. J Neurosurg. 2003;98(1):125–30. https://doi.org/10.3171/jns.2003.98.1.0125.
Acknowledgments
The authors gratefully acknowledge Dr. Sean McBride for his technical assistance, Ms. Julia Katris for contributions to the histological processing, and Dr. Amit Vasanji for providing the micro-CT data and analysis. The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Funding
This work was supported by the Army, Navy, NIH, Air Force, VA, and Health Affairs to support the AFIRM II effort under Award No. W81XWH-14-2-0003. The U.S. Army Medical Research Acquisition Activity, Fort Detrick, MD is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The current study was also supported by the National Institute Of Biomedical Imaging And Bioengineering of the National Institutes of Health under Award Number P41EB001046. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S.S., Ortiz, O., Pastino, A.K. et al. Hybrid Bone Scaffold Induces Bone Bridging in Goat Calvarial Critical Size Defects Without Growth Factor Augmentation. Regen. Eng. Transl. Med. 6, 189–200 (2020). https://doi.org/10.1007/s40883-019-00144-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00144-z