Abstract
Cadmium oxide (CdO) and chromium (Cr) doped CdO thin films with various Cr wt% contents (0.1, 0.5, 1, 2 at.%) are synthesised on soda lime glass (SLG) substrates. Results from X-ray analysis indicate that all thin films are in cubic nature with (111) preferential orientation. Cr doping does not appear to result in a transition from one crystal phase to another. CdO with the 2.0 wt% Cr doping absorbs much more light than undoped CdO in low wavelength region (< ≈450 nm), giving a maximum absorption peak in the ultraviolet region. Cr doped CdO/p-Si heterojunction shows the Cr wt% content-sensitive rectification property, achieving a high ratio of 105. In addition, Cr doped CdO/p-Si heterojunction exhibits photodiode behavior and possesses an improved photo-to-dark current ratio of ≈153. This work introduces an occasion to develop the optoelectronic devices based on Cr doped CdO thin films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transparent conducting oxides (TCOs) are one of the key components in optoelectronic devices such as solar cell, photovoltaics, organic light-emitting diode, flat panel liquid crystal display and optical waveguide [1,2,3,4,5]. Most of TCO’s are based on tin, zinc, cadmium, indium and titanium. Historically, cadmium oxide (CdO) is one of the first examples studied as TCO. It has attracted considerable attention due to reasons such as the simple crystal structure, easy preparation, high deposition rate to large surface, high transparency in visible region, metallic/optic conductivity, doping with various dopant materials (element), high carrier mobility, low reflection (anti-reflection coating), low electrical resistivity and large direct band gap (as optical window). Many techniques have been applied to fabricate CdO, i.e. sol–gel, chemical vapor deposition, electrodeposition, pulsed laser deposition [6], screen-printing [7], spray pyrolysis [8, 9], sputtering [10] and vacuum evaporation [11]. Sol–gel is preferred to other methods due to easy integration of CdO with various metallic ions. The characteristic properties of CdO could be governed by doping content and even further improved.
Chromium (Cr) is likely to exist in two valency states, i.e. Cr3+/Cr4+, it gets 6 + valency with the reduction reaction. The incorporation of Cr into TiO2 creates the newly formed Cr 2p level in the band gap and oxygen vacancies in lattice. The absorption increases with Cr doping in the visible range, demonstrating a redshift in the absorption edge. Cr doped TiO2 nanoparticles show reduced reflectance in the wavelength range of visible light due to the smaller sizes of the nanoparticles. Decreasing reflectance and increasing absorption are a practical way to promote the current conversion efficiency in photovoltaics, depending on a low light scattering capability and high light harvesting ability [12]. Doping with some elements paves the way two opposite effects on semiconductors, namely the band gap narrowing (BGN) or band gap widening. The researches show that the doping with Cr ions decreases the band gap of the ZnAl2O4 by about 12.5%, resulting in a redshifted absorption spectrum and band gap narrowing (BGN). With the increase in Cr content, the crystallite size of Zn(1−x)CrxAl2O4 (x = 0.00, 0.05, 0.10, 0.20) nanoparticles (NPs) decreases as well. This effect may be related to van der waals and coulomb forces that contribute to the agglomeration of NPs due to Cr doping [13]. Enhancement of dye degradation by transition-metal doped ZnO has been reviewed. The dye degradation performance of ZnO with various Cr doping concentrations is compared to pure ZnO. The formation of ZnCrO4 structure occurs in case of doping more than 6%, which reduces the dye degradation rate as the Cr atoms are not substituted into ZnO lattice as an interstitial atom [14, 15]. It is assumed that the ionic radius of the dopant ion should be close to/smaller than that of the element in the host lattice. Cr-cation (Cr3+) doping can easily replace into CdO lattice by substituting Cd2+ ion since Cr ions of radius 0.068 nm are smaller than Cd ions (0.097 nm) [16].
Chemical doping is one of the ways to increase the photoresponsivity of layered semiconductors [17]. Undoped CdO/p-Si heterojunction exhibited a weak current gain in Ref [18]. The photocurrent efficiency can be achieved by better transport properties of each contact component. The researches show that Cr can be used as a dopant to enhance the magnetic, photoluminance and optical properties [13]. Lack of data on Cr doped CdO is a motivation to synthesize Cr doped CdO, using sol–gel method. However, there is no previous report on the photoresponse properties and preparation of Cr doped CdO/p-Si heterojunction.
In this study, undoped CdO and Cr doped CdO thin films with various Cr doping concentrations (0.1, 0.5, 1.0 and 2.0%) are synthesized by sol–gel method and their characterizations are carried out by XRD and UV–Vis spectroscopy. Effect of Cr doping on the diode parameters of Cr doped CdO/p-Si heterojunction is investigated. Additionally, the photoresponse properties of the heterojunction diode are studied in detail.
2 Experimental details
Monoethanolamine (MEA) (C2H7NO), 2-methoxyethanol (C3H8O2), cadmium acetate dihydrate (CAD) (Cd(CH3COO)2.2H2O) are used as stabilizer, solvent and precursor material, respectively. The detailed synthesis of the CdO is given in our previous work [18]. Undoped CdO and CdO consisting of various Cr wt% contents (0.1, 0.5, 1, 2 at.%) were coated on the glass (soda lime glass) and p-type silicon (p-Si) substrates. Samples were annealed (heat treatment) at 500 °C. Highly pure Aluminum (Al) was thermally evaporated to the matte side of p-Si substrates and then exposed to a thermal treatment at 550 °C for 5 min in N2 atmosphere. Al was thermally evaporated through circular mask on p-Si substrate coated with CdO consisting of various Cr wt% contents. Finally, Al/Cr doped CdO/p-Si heterojunction structure was obtained (Fig. 1).
2.1 Characterization
UV–visible (UV–Vis) spectrophotometer (Shimadzu UV–VIS/NIR 3600) is a compact device and used for the reflectance, absorbance and transmittance data. The measurement was performed over the wavelength range 280 nm to 1100 nm. X-ray diffractometer (XRD, Rigaku Ultima IV) with CuKα radiation was used to obtain the crystallographic data of CdO thin films. The electrical characterization was carried out for the p-Si based heterojunctions. Keithley 2400 voltage source (Keithley's Standard Series 2400 Source Measure Unit (SMU)) was used as electrometer to analyse the current-voltage (I-V) characteristics of Cr doped CdO/p-Si heterojunctions. The light dependent on electrical characteristics of the heterojunction were measured by using a solar simulator system.
3 Results and discussion
3.1 Structural and optical characteristics
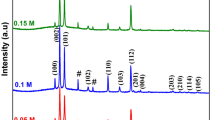
Figure 2 shows X-ray diffraction (XRD) pattern of undoped CdO and Cr doped CdO thin films deposited on glass substrates. The patterns were performed in 2θ range of 20–100°. Rietveld refinement of X-ray diffraction pattern was used for determining the structural information of CdO thin films. The first and second order Bragg's angles are ≈33° and ≈38°, corresponding to the (111) and (200) planes. Results indicate that undoped CdO and Cr doped CdO thin films are in cubic nature with (111) and (200) dominant orientation. Data collection obeys the card number of Joint Committee on Powder Diffraction Standard (JCPDS): 05-0640. The recorded patterns are consistent with the indexed planes of CdO. Also, crystalline orientations (220) and (311) are identified as distinct peaks in the spectra [19, 20]. Cr doping does not change the crystal phase of CdO (with the incorporation of Cr in the CdO lattice). The major diffraction peaks of CdO are still retained after incorporation with Cr.
There is a relation between the lattice constant (a) and Miller indices (hkl) dependent on interplanar spacing (d):
The values for the lattice constant and interplanar spacing of CdO thin films are given in Table 1 as a function of Cr content. The lattice constant determined from XRD data is in good agreement with the literature [21, 22]. The positions of the intense CdO (111) and (200) as ∆(2θ111 and 2θ200) peaks show a slight shift towards higher Bragg angle from 32.96° and 38.18° for undoped CdO to 33.02° and 38.52° for 0.1 wt% Cr doped CdO. A slight shift Δ(2θ) in the Bragg angle towards higher values is associated with a slight reduction in the lattice constant. Also, it is attributed to the structural strain (ε = − ∆θ(hkl)cotθ(hkl)) to be created owing to the doping of Cr, which has a smaller ionic radius compared to that of Cd2+ ion [23].
The crystallite size is estimated by using Scherrer’s formula as follows [24]:
where θ is the diffraction angle, β is the full width at half maximum (FWHM) for the diffraction peak, k is called the shape factor and a dimensionless constant (k = 0.94). λ is the wavelength of X-ray (λ = 1.54056 Å). β is corrected by taking into account the instrumental broadening [25]:
The crystallite size for the samples is given in Table 1. It is seen that the crystallite size decreases with the incorporation of Cr into CdO. The result is consistent with that of ZnO prepared by doping with Co, which is a transition metal like Cr [26]. The reduction in the crystallite size is elucidated by considering difference between ionic radii of Cr and the Cd ions, that is, the size difference of the Cr ions (0.068 nm) and Cd ions (0.097 nm) [16].
Considering that X-ray diffraction contains several peaks, Williamson-Hall (W-H) method can be approached as an alternative and more accurate method to calculate the crystallite size [27].
where λ, β, k and θ have the same meanings as given in the Scherrer’s method. Plotting the β cosθ versus 4sinθ to get a straight line with slope ε and intercept 0.9λ/D gives the strain and average crystallite size values (Fig. 3). The crystallite size and the average microstrain values of undoped CdO and Cr doped CdO thin films with various Cr wt% contents (0.1, 0.5, 1, 2 at.%) are listed in Table 1.
Dislocation density (δ) in the unity of lines/m2 and the microstrain (ε) are given as follows [23]:
where n is a factor and it equals to unity at minimum value of dislocation density. The δ and ε values are given in Table 1. The dislocation density (δ) is in the order of 1015 lines/m2 for all samples, indicating an agreement with the dislocation density of CdO thin films obtained by spray pyrolysis in Ref [28]. The microstrain (ε) formed by the dopant element represents the actual contribution of Cr to the host CdO crystallite. The increase in the ε is due to the increased aggregation of Cr around the crystallite boundary. The stacking fault (αst) is computed from X-ray peak shift measurements in terms of probability [29]:
The stacking fault probability ranges from 0.102 to 0.114 for undoped CdO and Cr doped CdO samples (as 0.103, 0.102, 0.114, 0.112 and 0.106, respectively). The dislocation density can also be evaluated together with the stacking fault.
The preferred orientation of the crystalline thin films is evaluated through the texture coefficient (TChkl). It is formulated as follows [30]:
where N represents the number of diffraction peaks, I(hkl) is the measured relative intensity of the plane (hkl). Io(hkl) is the standard intensity of the plane (hkl). The texture coefficients for CdO and Cr doped CdO thin films are given in Table 1. The texture coefficient close to unity is related to the randomness of crystal orientation [31]. The TC(hkl) value of peak (111) decreases with the incorporation of Cr. Increasing the TC(hkl) causes grain boundary mismatch, which further increases porosity in thin film [32].
Transmittance (T) allows to determine the band gap energy (Eg) of transparent conductive oxides (TCOs). Figure 4a shows the optical transmittance measurement of undoped CdO and Cr doped CdO thin films in the wavelength range of 280–1000 nm. The transmittance shows two different discontinuities (absorption edge) one at ≈300 nm and the other at ≈500 nm. At low wavelength region (< 400 nm), the transmittance decreases with the doping of Cr, while undoped CdO gives the highest value. However, the change in transmittance reverses at longer wavelengths, exhibiting the highest transparency for the 2 wt% Cr doping. The transmittance is low when compared to that of Al doped CdO films (92–96%) [19].
The absorption edge is known as absorption limit or absorption discontinuity and corresponds to the band gap energy (Eg). The highest absorption is observed for CdO films with 2% Cr (Fig. 4b). The optical band gap can be determined by using Tauc equation for a semiconductor with the direct band gap [33].
where α(= (1/d)ln(1/T), T transmittance, d thickness) is the absorption coefficient and A is a constant. Figure 4c shows the graph of (αhυ)2 as a function of photon energy for undoped CdO and Cr doped CdO thin films. The optical band gap determined by extrapolating the linear part of the plot is given in Table 1. The oxygen vacancy (Vo) in oxide systems can act as donor by creating new energy level in the band gap, and thus the Vo may be served on the BGN [34]. Oxygen vacancy with unpaired electron facilitates more oxygen adsorption on the surface by supplying gas adsorption and reaction. Cr doping could be a promising method to improve the gas detection of optoelectronic sensing devices [35]. The shift of absorption edge with the doping of semiconductor is attributed to the Burstein-Moss effect [36]. Hymavathi et al. [15] obtained Cr doped CdO thin films by reactive direct current magnetron sputtering. The band gap determined in the range of 2.65–2.77 eV as a function of annealing temperature is higher than the values estimated in the current study. It is known that the incorporation of Cr into ZnO increases the defect level, facilitates the generation of electron-hole pairs (e)-(h) and narrows the band gap. Cr incorporation, that contributes to a shift in the absorption edge toward higher wavelengths causes a decrease in the band gap compared to undoped sample [37].
The band edge does not show energetically regular line in the semiconductor. The energetic disorder is characterized by Urbach energy (Eu), which depends exponentially on the absorption coefficient [38].
where α0 is a constant in the inverse length dimension and also a fitting parameter. Plotting of lnα vs. \(h\nu\) is shown in Fig. 5. The Eu is determined from the slope of a straight line region:
It is seen that the Eu for CdO thin films decreases with Cr doping (Table 1). This implies that Cr doping causes a reduction in the structural defects of CdO thin films.
Figure 6 shows the reflectance (R) characteristics for undoped CdO and Cr doped CdO thin films. While the reflectance does not change in the ultraviolet (UV) region, it shows a significant change in the visible region. The reflectance values of CdO thin films are low compared to Cr doped samples (in the range of 460–700 nm). The reflectance of 0.5% Cr doped CdO thin films has the highest value, attributing to scattering at the grain boundary.
The complex refractive index, which is sum of the real and imaginary parts is as follows [39]:
where k and n are the extinction coefficient and the refractive index, respectively. Figure 7a, b shows the refractive index and extinction coefficient versus wavelength (λ), respectively. The origin of the change (in the plotting n vs. λ) is band-to-band transition due to the optical excitation. The large value of k specifies the fundamental band gap of the semiconductor at short wavelengths [40]. While the refractive index (n) changes at long wavelengths, as in reflectance, the exciton coefficient gives a peak at short wavelengths. It is concluded that Cr doping in CdO is dominant on the change of exciton coefficient. The dispersion dependent on refractive index provides more information about crystal optics. The refractive index dispersion is evaluated by considering the single oscillator model based on Wemple–DiDomenico relationship [41, 42]:
where average excitation energy/the single-oscillator energy and the dispersion energy are represented by E0 and Ed, respectively. Dispersion model is described by the dielectric response function of the material. The parameters are related to the excitation energy and the intensity of inter-band transition and determined by plotting (n2 − 1)−1 versus (\(h\nu\))2 (Fig. 8). The plotting gives a straight line including the intercept (E0/Ed) and slope (1/E0Ed). The values determined are summarized in Table 1. It is seen that the average excitation energy/the single-oscillator energy and the dispersion energy values are influenced by Cr doping, giving higher values for undoped sample.
3.2 The current-voltage (I-V) characteristics of Cr doped CdO/p-Si heterojunction diodes
Figure 9a shows the current–voltage (I-V) characteristics of p-Si based heterojunctions with Cr doped CdO thin films. The turn-on voltage is in the range of ≈0.3–0.5 V. The reverse bias current depends on strongly the Cr wt% content. The leakage current density is 6.76 × 10–8 A/cm2 for the sample with the 0.1 wt% Cr and considerably smaller than that of the p-Si junction based on CdO doped with Mn, which is a transition metal like Cr [43]. Cr doped CdO/p-Si heterojunctions show rectifying behavior. The rectification ratio (RR(V) =|J(V)/J(− V)|, J = current density in A/cm2) is as large as 4.37 × 105 at ± 2 V for the diode with the 0.1 wt% Cr (ultrahigh-rectification ratio). It is found that the doping profile creates a remarkable rectifying effect. The rectification performance for σ − π-type single-molecule diode is related to the metal used as the electrode. A significant rectifying effect is attributed to the Pt electrode used instead of Ag, since the tunneling electrons, which increase the forward bias current, are closer to the Fermi level of the Pt electrode [44].
The characteristic curve of a junction diode deviates from ideal behavior due to the series resistance and junction defects [45]. The current density equation is as follows:
The heterojunction is non-ideal diode with an ideality (n) factor. It is considered that the conduction mechanism is dominated by thermionic emission (TE) over the potential barrier.
where \(\phi_{b}\) is the barrier height. J and J0 are the current density (measured) and the saturation current density, respectively. J0 is determined by extrapolating J-V (semi-log) plotting to V = 0. The effective Richardson constant (A*) is 32 A/cm2K2 for p-Si. Ideality factor was determined to be 1.2 for the diode with the 0.1 wt% Cr doping. Due to the Cr-dopant-governed behavior of the CdO, the diode parameters can be modulated by the components of heterojunction. However, a high ideality factor of 5.41 is determined for nanocluster n-CdO/p-Si heterojunction diode, attributing to the presence of the series resistance and interface states [46]. It is reported that Ag/Cu:CdO/p-Si and Ag/Al:CdO/p-Si diodes have ideality factor around 3.87 to 4.84. Additionally, the existence of native oxide layer on the Si substrate also results in higher ideality factor [47]. The \(\phi_{b}\) values for the 0.1, 0.5 and 1.0 wt.% Cr doping are determined to be 0.86 eV, 0.65 eV and 0.84 eV, respectively (Table 2). In our previous work, the values of n and \(\phi_{b}\) for undoped CdO/p-Si diode are found to be 1.43 and 1.03 eV, respectively, [18]. This indicates that Cr doping is favorable for the control of barrier properties.
Norde defined a function (F(V)) for the barrier height (\(\phi_{b}\)) and series resistance (Rs) [48]:
where \(\gamma\) is the integer (dimensionless) greater than n and the Vo the minimum voltage. Figure 9b shows the plots of F(V) as a function of voltage. The \(\phi_{b}\) and Rs can be determined by using the F(Vo) and Io values, respectively. The values of the \(\phi_{b}\) for the 0.1, 0.5 and 1.0 wt% Cr doping are 0.92 eV, 0.67 eV and 0.87 eV, respectively. Norde function provides an evaluation of all data (whole forward I-V). Therefore, the values obtained are larger than those determined by thermionic emission theory (TET). Also, it is seen that the Rs increases with increasing Cr content, attributing to the interface states, defect states, charges trapped at the interface and oxide layer on Si substrate (Table 2).
The electrical characteristics of 0.1 wt% Cr doped CdO/p-Si diode with low leakage current value have been studied at 20 mW/cm2 steps up to 100 mW/cm2 under the light (Fig. 10). The heterojunction diode shows a rectifying property in the existence of illumination too. The heterojunction exhibits photodiode behavior. While the photodiode shows the reverse dark current density of 67.6 nA/cm2 at 2 V, it exhibits the reverse bias current density of 4.24 μA/cm2 and 10.42 μA/cm2 for 20 mW/cm2 and 100 mW/cm2 light intensity, respectively. CdO layer between p-Si and metal also acts to reduce the reflection (antireflection). Photons pass through CdO thin films. The photon-absorption dependent on generation current (electron-hole pairs) relates to the depletion region of p-Si. Photons have a depth of penetration on the semiconductor (p-Si). If the photons can not penetrate through the whole depletion layer of the semiconductor, the current reaches its saturation value. The total current is the current which originates from the electrons and holes:
where ∆pph and ∆nph are the average hole density and electron density produced by photon, Vn,p is the average drift velocity of carriers. Figure 11 shows the energy band diagram of the CdO/p-Si heterojunction. In the case of the reverse bias, the photogenerated electrons are transferred over the depletion layer of CdO towards the Al contact on the CdO thin film, while the holes are swept over the depletion layer of CdO towards the Al back contact under the light. It is likely that there is dangling bond on the surface of the Si wafer. Therefore, it is inevitable for Si atoms to bond with oxygen (O2) atoms, usually by forming silicon dioxide (SiO2 as a thin layer). Photoelectrons traveling towards the Al contact on the CdO are blocked by the SiO2 layer at the CdO/p-Si interface. However, there is no such a barrier for the photogenerated holes that go to the Al back contact. So, the Jph is domitated by the photogenerated holes in the depletion region of p-Si and the photoelectrons in the depletion region of n-CdO [49].
There is a relationship between the photocurrent and illumination intensity (P) as the power law distribution [50]:
where, α is the illumination coefficient (the power law exponent) to be the empirical value (in the range < 0.5, 0.5–1 and > 1). It is a scale of the photocurrent's response to illumination intensity (P), relating to the recombination mechanism of the photogenerated charge. Figure 12 shows the plot of Iph vs. P in log scale form. The value of α is evaluated to study the photoconductivity of Cr doped CdO/p-Si. It is estimated to be the α of about 0.54. This indicates improved carrier transport and the presence of persistent localized states [51]. The value of the α close to 1 indicates that recombination is negligible as a loss process [52].
The photoresponsivity (R) for the photovoltaics is used to test and interpret the light sensitivity (R = Jph/P). Also, the photosensitivity (photo-to-dark current Id, η) is identified with η = (Iph)/Idark. Figure 13 shows the variation of R and η as a function of the incident light for CdO/p-Si photodiode with 0.1 wt.% Cr doped CdO layer. It is observed that the photoresponsivity decreases with increasing light intensity (in steps of 20 mW/cm2). The decrease of the responsivity at high light intensity is explained in terms of the electron-trap saturation and increase of electron-hole recombination. As the excited electrons fill the traps, the quasi-Fermi energy level increases and the system contains a large number of free electrons [53]. The photosensitivity is found to be 153.12 for the power density of 100 mW/cm2 (Fig. 13). It is seen that the photosensitivity increases with increasing light intensity. Furthermore, detectivity (D*, in Jones) is defined as D* = R/(2qJd)1/2 = (Jph/P)/(2qJd)1/2, considering the noise current and dark current. The shot noise is responsible for minimum dark current (Id) in the detectivity of the photodiode, consisting of the statistical fluctuation in the Iph and Id [54, 55]. CdO/p-Si device with 0.1 wt% Cr doped CdO layer achieved D* = 1.41 × 109 Jones. The detectivity of silicon photodiode based on CdO thin film is comparable to polymer photodetector with the ITO/PEDOT/PDDTT:PC60BM/A1 junction structure [55].
To analyse the illumination intensity range, another important parameter for photovoltaics is the linear dynamic range (LDR, in units of dB). This is also known as photo-sensitivity linearity and given as:
The photoresponsivity shows a linear regime depending on the incident light intensity. When Cr doped CdO/p-Si diode is operated under the incident light power of 100 mW/cm2, the LDR gives 43.7 dB, which is comparable to InGaAs photodetector (66 dB).
Noise equivalent power (NEP) is defined to distinguish the sensitivity of photodetector from a noise source and given as [56]:
∆f is the electrical bandwidth in Hz. A is the effective area (0.0314 cm2). The minimum value for the NEP is 0.0124 pW Hz−1/2, which is lower than that obtained from graphene/n-Si heterojunction photodetector [52]. The detector shows the linearity with the minimum impinging optical power due to small NEP and noise current.
4 Conclusions
Undoped CdO and Cr doped CdO were coated onto soda lime glass substrates by cost-effective and easy method (sol–gel). All the thin films are poly-crystalline in cubic nature with (111) preferential orientation. It is seen that Cr doping does not change the crystalline structure of CdO. The optical parameters such as the average excitation energy/the single-oscillator energy and the dispersion energy appear to depend on the Cr wt% content in CdO, giving higher values in undoped sample. Al/CdO/p-Si heterojunction structure exhibited a rectifying performance sensitive to the Cr wt% content, giving a high ratio of 105. In addition, Cr doped CdO/p-Si heterojunction possesses the ability to detect weak light signals (detectivity) and an improved photo-to-dark current ratio of ≈153, exhibiting the photodiode behavior. The fabricated heterojunction structure could introduce a great occasion for opening avenues to new photovoltaic applications.
Data availability
Data available on request from the author.
References
B.J. Ingram, G.B. Gonzalez, D.R. Kammler, M.I. Bertoni, T.O. Mason, Chemical and structural factors governing transparent conductivity in oxides. J. Electroceram. 13, 167–175 (2004). https://doi.org/10.1007/s10832-004-5094-y
C.G. Granqvist, A. Hultaker, Transparent and conducting ITO films: new developments and applications. Thin Solid Films 411, 1–5 (2002). https://doi.org/10.1016/S0040-6090(02)00163-3
S. Sindhu, K.N. Rao, S. Ahuja, A. Kumar, E.S.R. Gopal, Spectral and optical performance of electrochromic poly(3,4-ethylenedioxythiophene) (PEDOT) deposited on transparent conducting oxide coated glass and polymer substrates. Mater. Sci. Eng. B 132, 39–42 (2006). https://doi.org/10.1016/j.mseb.2006.02.030
R.G. Gordon, Criteria for choosing transparent conductors. MRS Bull. 25, 52–57 (2000). https://doi.org/10.1557/mrs2000.151
K.M. Yu, M.A. Mayer, D.T. Speaks, H. He, R. Zhao, L. Hsu, S.S. Mao, E.E. Haller, W. Walukiewicz, Ideal transparent conductors for full spectrum photovoltaics. J. Appl. Phys. 111, 123505-1–123505-5 (2012). https://doi.org/10.1063/1.4729563
E. Li, H. Zhuo, H. He, N. Wang, T. Liu, Structural, optical, and electrical properties of low-concentration Ga-doped CdO thin films by pulsed laser deposition. J. Mater. Sci. Mater. Electron. 51, 7179–7185 (2016). https://doi.org/10.1007/s10853-016-9998-0
V. Kumar, R. Kumari, D.K. Sharma, K. Sharma, S. Shukla, A. Agrwal, Structural, optical, and electrical characterization of Sol-Gel processed screen-printed CdO: Li Film. J. Appl. Spectrosc. 90, 151–154 (2023). https://doi.org/10.1007/s10812-023-01516-8
M. Anitha, K.S. Kumar, N. Anitha, I. Kulandaisamyd, L. Amalraj, Influence of annealing temperature on physical properties of Sn-doped CdO thin films by nebulized spray pyrolysis technique. Mater. Sci. Eng. B 243, 54–69 (2019). https://doi.org/10.1016/j.mseb.2019.03.018
K. Sankarasubramanian, M. Sampath, J. Archana, K. Sethuraman, K. Ramamurthi, Y. Hayakawai, Influence of substrate temperature on ethanol sensing properties of CdO thin films prepared by facile spray pyrolysis method. J. Mater. Sci. Mater. Electron. 26, 955–961 (2015). https://doi.org/10.1007/s10854-014-2488-8
B. Saha, R. Thapa, K.K. Chattopadhyay, Wide range tuning of electrical conductivity of RF sputtered CdO thin films through oxygen partial pressure variation. Sol. Energy Mater. Sol. Cells 92, 1077–1080 (2008). https://doi.org/10.1016/j.solmat.2008.03.024
F.C. Eze, Oxygen partial pressure dependence of the structural properties of CdO thin films deposited by a modified reactive vacuum evaporation process. Mater. Chem. Phys. 89, 205–210 (2005). https://doi.org/10.1016/j.matchemphys.2003.11.039
R.S. Dubey, S. Singh, Investigation of structural and optical properties of pure and chromium doped TiO2 nanoparticles prepared by solvothermal method. Res. Phys. 7, 1283–1288 (2017). https://doi.org/10.1016/j.rinp.2017.03.014
M. Nadafan, G.H.H. Khorrami, M. Arab, Z. Dehghani, A. Kompany, Influence of Cr doping on the structural, optical, and third-order nonlinear optical properties of ZnAl2O4 nanoceramics. AIP Adv. 13, 055122 (2023). https://doi.org/10.1063/5.0147932
M.A. Moiz, A. Mumtaz, M. Salman, H. Mazhar, M.A. Basit, S.W. Husain, M. Ramzan, Enhancement of dye degradation by zinc oxide via transition-metal doping: a review. J. Electron. Mater. 50, 5106–5121 (2021). https://doi.org/10.1007/s11664-021-09093-2
A. Meng, J. Xing, Z. Li, Q. Li, Cr-doped ZnO nanoparticles: synthesis, characterization, adsorption property, and recyclability. ACS Appl. Mater. Interfaces 7, 27449–27457 (2015). https://doi.org/10.1021/acsami.5b09366
B. Hymavathi, B.R. Kumar, T.S. Rao, Post-annealing effects on surface morphological, electrical and optical properties of nanostructured Cr-doped CdO thin films. J. Electron. Mater. 47, 503–511 (2018). https://doi.org/10.1007/s11664-017-5799-8
S. Ghosh, A. Varghese, K. Thakar, S. Dhara, S. Lodha, Enhanced responsivity and detectivity of fast WSe2 phototransistor using electrostatically tunable inplane lateral p-n homojunction. Nat. Commun. 12, 3336 (2021). https://doi.org/10.1038/s41467-021-23679-8
M. Soylu, H.S. Kader, Photodiode based on CdO thin films as electron transport layer. J. Electron. Mater. 45, 5756 (2016). https://doi.org/10.1007/s11664-016-4819-4
B.K. Balachandar, T. Logu, R. Hari Ramprasath, K. Sankarasubramanian, P. Soundarrajan, M. Sridharan et al., Spray pyrolysis deposited CdO: Al films for trimethylamine sensing application. Mater. Sci. Semicond. Process. 105, 104753 (2020). https://doi.org/10.1016/j.mssp.2019.104753
M. Ramamurthy, M. Balaji, P. Thirunavukkarasu, Characterization of jet nebulizer sprayed CdO thin films for solar cell application. Optik 127, 3809–3819 (2016). https://doi.org/10.1016/j.ijleo.2016.01.031
T.K. Subramanyam, S. Uthanna, B.S. Naidu, Preparation and characterization of CdO films deposited by dc magnetron reactive sputtering. Mater. Lett. 35, 214–220 (1998). https://doi.org/10.1016/S0167-577X(97)00246-2
K.T.R. Reddy, C. Sravani, R.W. Miles, Characterisation of CdO thin films deposited by activated reactive evaporation. J. Cryst. Growth 184(185), 1031–1034 (1998). https://doi.org/10.1016/S0022-0248(98)80215-7
A.A. Dakhel, Transparent conducting properties of samarium-doped CdO. J. Alloys Compd. 475, 51 (2009). https://doi.org/10.1016/j.jallcom.2008.08.008
R. Ghosh, D. Basak, S. Fujihara, Effect of substrate-induced strain on the structural, electrical, and optical properties of polycrystalline ZnO thin films. J. Appl. Phys. 96, 2689–2692 (2004). https://doi.org/10.1063/1.1769598
M.M. Mahasen, M.M. Soraya, E.L.S. Yousef, G.A.M. Ali, E.R. Shaaban, Structural, thermal and optical analyses of cobalt-doped CdO thin films. J. Ovonic Res. 15, 247–260 (2019)
E.R. Shaaban, M. El-Hagary, M. El Sayed, H. Shokry Hassan, Y.A.M. Ismail, M. Emam-Ismail, A.S. Ali, Structural, linear and nonlinear optical properties of co-doped ZnO thin films. Appl. Phys. A 122, 20 (2016). https://doi.org/10.1007/s00339-015-9551-z
F. Abrinaei, S. Aghabeygi, Optimization on preparation conditions to improve the nonlinear optical response of ZnO/TiO2/ZrO2 ternary nanocomposites under continuous-wave laser irradiation. Optik 255, 168720 (2022). https://doi.org/10.1016/j.ijleo.2022.168720
K. Gurumurugan, D. Mangalaraj, S.K. Narayandass, Structural characterization of cadmium oxide thin films deposited by spray pyrolysis. J. Cryst. Growth 147, 355–360 (1995). https://doi.org/10.1016/0022-0248(94)00634-2
B.E. Warren, E.P. Warekois, Stacking faults in cold worked alpha-brass. Acta Met. 3, 473–479 (1955). https://doi.org/10.1016/0001-6160(55)90138-3
B. Benhaoua, A. Rahel, S. Benramache, The structural, optical and electrical properties of nanocrystalline ZnO: Al thin films. Superlatt. Microstruct. 68, 38–47 (2014). https://doi.org/10.1016/j.spmi.2014.01.005
D. Fang, K. Lin, T. Xue, C. Cui, X. Chen, P. Yao, H. Li, Influence of Al doping on structural and optical properties of Mg–Al co-doped ZnO thin films prepared by sol–gel method. J. Alloys Compd. 589, 346–352 (2014). https://doi.org/10.1016/j.jallcom.2013.11.061
M. Kumar, A. Kumar, A.C. Abhyankar, Influence of texture coefficient on surface morphology and sensing properties of W-doped nanocrystalline tin oxide thin films. ACS Appl. Mater. Interfaces 7, 3571–3580 (2015). https://doi.org/10.1021/am507397z
L.W. Wang, F. Wu, D.X. Tian, W.J. Li, L. Fang, C.Y. Kong, M. Zhou, Effects of Na content on structural and optical properties of Na-doped ZnO thin films prepared by sol–gel method. J. Alloys Compd. 623, 367–373 (2015). https://doi.org/10.1016/j.jallcom.2014.11.055
F. Gu, C. Li, D. Han, Z. Wang, Manipulating the defect structure (Vo) of In2O3 nanoparticles for enhancement of formaldehyde detection. ACS Appl. Mater. Interfaces 10, 933–942 (2018). https://doi.org/10.1021/acsami.7b16832
J. Sun, Y. Wang, P. Song, Z. Yang, Q. Wang, Metal-organic framework-derived Cr-doped hollow In2O3 nanoboxes with excellent gas-sensing performance toward ammonia. J. Alloys Compd. 879, 160472 (2021). https://doi.org/10.1016/j.jallcom.2021.160472
S.N. Svitasheva, A.M. Gilinsky, Influence of doping level on shift of the absorption edge of gallium nitride films (Burstein-Moss effect). Appl. Surf. Sci. 281, 109–112 (2013). https://doi.org/10.1016/j.apsusc.2013.02.094
H.M. Naeem, S. Tariq, M.A. Moiz, M. Khan, N. Rehman, M.A. Basit, Activating ZnO-based hierarchical particles for visible light dependent photocatalytic performance via Cr-incorporated rapid chemical synthesis. Cryst. Res. Technol. 57, 2100125 (2022). https://doi.org/10.1002/crat.202100125
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324 (1953). https://doi.org/10.1103/PhysRev.92.1324
A. El-Korashy, H. El-Zahed, M. Radwan, Optical studies of [N(CH3)4]2CoCl4, [N(CH3)4]2MnCl4 single crystals in the normal paraelectric phase. Physica B 334, 75–81 (2003). https://doi.org/10.1016/S0921-4526(03)00019-X
J. Rodriguez, M. Gomez, J. Ederth, G.A. Niklason, C.G. Granqvist, Thickness dependence of the optical properties of sputter deposited Ti oxide films. Thin Solid Films 365, 119–125 (2000). https://doi.org/10.1016/S0040-6090(99)01109-8
M. DiDomenico, S.H. Wemple, Oxygen-octahedra ferroelectrics. I. Theory of electro-optical and nonlinear optical effects. J. Appl. Phys. 40, 720–734 (1969). https://doi.org/10.1063/1.1657458
S.H. Wemple, M. DiDomenico, Behavior of the electronic dielectric constant in covalent and ionic materials. Phy. Rev B. 3, 1338–1351 (1971). https://doi.org/10.1103/PhysRevB.3.1338
S. Dugan, M.M. Koc, B. Coskun, Structural, electrical and optical characterization of Mn doped CdO photodiodes. J. Mol. Struct. 1202, 127235 (2020). https://doi.org/10.1016/j.molstruc.2019.127235
G.P. Zhang, L.Y. Chen, J.M. Zhao, Y.Z. Sun, N.P. Shi, M.L. Wang et al., Large rectification ratio of up to 106 for conjugation-group-terminated undecanethiolate single-molecule diodes on Pt electrodes. J. Phys. Chem. C 125, 20783–20790 (2021). https://doi.org/10.1021/acs.jpcc.1c04093
K.W. Boer, Nonideal diode factors. J. Appl. Phys. 51, 4518–4522 (1980). https://doi.org/10.1063/1.328393
F. Yakuphanoglu, M. Caglar, Y. Caglar, S. Ilican, Electrical characterization of nanocluster n-CdO/p-Si heterojunction diode. J. Alloys Compd. 506, 188–193 (2010). https://doi.org/10.1016/j.jallcom.2010.06.174
P. Umadevi, N. Prithivikumaran, Electrical parameters of metal doped n-CdO/p-Si heterojunction diodes. Physica B 501, 123–128 (2016). https://doi.org/10.1016/j.physb.2016.08.002
H. Norde, A modified forward I-V plot for Schottky diodes with high series resistance. J. Appl. Phys. 50, 5052–5053 (1979). https://doi.org/10.1063/1.325607
I.-S. Jeong, J.H. Kim, S. Im, Ultraviolet-enhanced photodiode employing n-ZnO/p-Si structure. Appl. Phys. Lett. 83, 2946–2948 (2003). https://doi.org/10.1063/1.1616663
R.H. Bube, Photoconductivity of Solids (Wiley, New York, 1960)
A. Rose, Concepts in Photoconductivity (Interscience, NewYork, 1963)
X. Li, M. Zhu, M. Du, Z. Lv, L. Zhang, Y. Li et al., High detectivity graphene-silicon heterojunction photodetector. Small 12, 595–601 (2016). https://doi.org/10.1002/smll.201502336
C. Lan, C. Li, Y. Yin, H. Guo, S. Wang, Synthesis of single-crystalline GeS nanoribbons for high sensitivity visible-light photodetectors. J. Mater. Chem. C 3, 8074–8079 (2015). https://doi.org/10.1039/C5TC01435B
M.M. Shahidi, M.H. Ehsani, H.R. Dizaji, M.E. Ghazi, Photoresponsivity enhancement of SnS porous film. Surf. Interfaces 21, 100790 (2020). https://doi.org/10.1016/j.surfin.2020.100790
X. Gong, M. Tong, Y. Xia, W. Cai, J.S. Moon, Y. Cao et al., High-detectivity polymer photodetectors with spectral response from 300 nm to 1450 nm. Science 325, 1665–1667 (2009). https://doi.org/10.1126/science.1176706
L. Dou, Y. Yang, J. You, Z. Hong, W.H. Chang, G. Li, Y. Yang, Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 5, 5404 (2014). https://doi.org/10.1038/ncomms6404
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No funding was received during this study.
Author information
Authors and Affiliations
Contributions
MS: Investigation, original draft, Methodology, Formal analysis, Writing, review and editing.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soylu, M. Effect of Cr doping and photoresponse properties of photodiode based on CdO thin films. J Mater Sci: Mater Electron 35, 771 (2024). https://doi.org/10.1007/s10854-024-12529-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12529-0