Abstract
Nanoparticles of Pr0.6-xBixSr0.4MnO3 (x = 0.10 and 0.25) have been prepared using the swift and cost-effective top-down approach in a high-energy planetary ball mill without any chemical processing and environmental impacts resulting due to pollutants arising from nanoparticle synthesis. The work investigates the role of particle size reduction on the structural and magnetic properties. An increase in milling time from 0 to 240 min results in the drastic reduction of particle size which drops significantly in the initial phase of ball milling i.e., 0–60 min while the microstrain and dislocation densities show nearly monotonic increase. Interestingly the magnetism and size reduction demonstrate a clear one to one correspondence. In both compositions, the paramagnetic (PM) to ferromagnetic (FM) transition, TC decreases and the magnetic transitions broaden both as function Bi and size reduction. The Curie–Weiss analysis of the inverse magnetic susceptibility (\({\chi }^{-1}\)) shows a deviation from linearity indicating the presence of the short-range FM interactions above TC which has been understood as Griffith’s phase like singularity. The size reduction can effectively reduce TC, enabling a significant control over tuning of transition temperature and net magnetization. The field dependent magnetization for x = 0.10 demonstrate dominant FM state whereas for x = 0.25, metamagnetic magnetization highlights competitive coexistence of FM and antiferromagnetic (AFM) interactions in the system. With ball milling, the net magnetization decreases, and the metamagnetic behaviour is suppressed. The M–H loops of ball milled nanoparticles are highly unsaturated indicating the enhanced surface disorder in the nanoparticles which can be understood using the core–shell structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanosized magnetic materials are of significant interest not only because of their potential applications in magnetic memory sensors and devices, magnetic refrigeration, ferrofluids, and biomedical applications such as magnetic drug delivery, magnetic imaging, magnetic hyperthermia, etc. but also for the wealthy scientific knowledge that arises due to quantum confinement and interparticle interactions [1,2,3,4]. With an increased concern towards the environmental pollution and carban foot prints a significant attention is being paid toward the fabrication nano particles for phtocalaysis and hydrogen evolution hand in hand processes[5,6,7]. The magnetic properties of nanoparticles are strongly influenced by the size, shape, and distribution of particles as well as the exchange interaction between them. Generally, when the size of the magnetic material is reduced to a few tens of nanometers, they show extraordinary physical properties such as low field magnetoresistance, large coercivity, and surface spin glass behaviour [1, 2]. With a further decrease in particle size (smaller than the magnetic domain size), the noninteracting particles result in superparamagnetism [8]. Realizing these outstanding properties that are sensitive to particle size reduction, magnetic nanoparticles are bringing revolutionary changes in a wide variety of applications.
The fabrication of nanoparticles can follow two pathways, viz. Top-Dowm (TD) approach and Bottom-Up (BU) approach each having its own merits and demerits. The former enables size reduction from bulk form to nanosize effortlessly and without any chemical biproducts. The lack of control over the shape and uniformilty in size is downside of this approach. The latter is inverse of TD method, where nano material of well-defined shape, size, and chemical composition are grown from self-assembled of atoms and molecules acting as a building blocks of nanomaterial however the evolution of harmful gases and toxic chemical recedues are major concerns to deal with. To name few of these routes, ballmilling, thermal evaporation, laser ablation, sonochemical and sputtering fall under TD method, the solgel, hydrothermal, coprecipitation and chemical vapour deposition are categorised unser BU method[9].
Among the magnetic materials, perovskite structured hole-doped manganites are best known for their colossal magnetoresistance (CMR), and magnetic/electronic phase separation that results because of the interaction between the spin, charge, and lattice degree of freedom [10,11,12]. These materials show interesting physical properties such as coexisting electrical and magnetic transitions, charge, orbital, spin ordering, multiferroicity, etc. that has resulted in the development of double-exchange, super-exchange model, and Jahn–Teller effect to explain the observed phenomena [10,11,12]. Currently, the investigation of the magnetic properties of nanocrystalline manganites has drawn great interest. Particularly the melting of the charge ordered (CO) state [13, 14], the influence of spin/structure disorder on the magnetism [15, 16], spin electronics based on half-metallic properties, and promising application in biology and medicine [17, 18] are matters of extensive discussion.
Out of all manganites, polycrystalline Pr1-xSrxMnO3 are interesting as they show a variety of magnetically ordered phases from A-type antiferromagnetic (AFM) insulator (x < 0.10) to canted AFM (x = 0.10) to ferromagnetic (FM) insulator (0.1 ≤ x ≤ 0.25) to ferromagnetic metallic (0.25 ≤ x ≤ 0.50) sensitive to Sr2+ concentration [19,20,21]. For x = 0.40 substitution of Sr2+, i.e., Pr0.6Sr0.4MnO3 (PSMO) is a room temperature metallic FM with Curie temperature, TC = 310 K and shows a moderate magnetoresistance (MR) of 40% and magnetic entropy change (∆S) of 3.41 J/kg-K across TC [22,23,24]. On the other hand, the family of bismuth based manganites (Bi1-xSrxMnO3 and Bi1-xCaxMnO3) has garnished the utmost attention for their CO state usually above room temperature [25,26,27,28]. Though Bi has a stable 3 + oxidation state and ionic size similar to that of Pr3+ [29], the physical properties of Bi1-xSrxMnO3 are distinct compared to Pr1-xSrxMnO3. The magnetic phases in Bi1-xSrxMnO3 vary from FM insulating (x = 0) to CO-AFM (0.30 ≤ x ≤ 0.80) [25]. For x = 0.40 substitution of Sr2+, i.e., Bi0.6Sr0.4MnO3 (BSMO) is a robust CO system with charge temperature TCO ≈ 600 K and AFM Néel temperature TN = 150 K. The contrasting properties of PSMO and BSMO, stimulate curiosity to explore the physical properties of intermediate compositions. Our systematic investigation on the structural and physical properties of Pr0.6-xBixSr0.4MnO3 (PBSMO) indicates a transition in the crystal symmetry from orthorhombic, space group Pnma for x ≤ 0.20 to Imma for x ≥ 0.50 with a structural phase coexistence corresponding to Pnma and Imma for 0.25 ≤ x ≤ 0.40. Correspondingly the magnetic state transforms from FM metallic for x ≤ 0.10 to AFM insulating for x ≥ 0.50 with magnetic phase coexistence for 0.20 ≤ x ≤ 0.40 [30]. In this paper, we report a comprehensive study of the effect of particle size reduction on the magnetic properties of Pr0.6-xBixSr0.4MnO3, x = 0.10 (i.e., PB10SMO) and x = 0.25 (i.e., PB25SMO). Since polycrystalline bulk PB10SMO represents a case with dominant FM state and in PB25SMO a competitive coexistence of FM and AFM interactions is seen, the present study will help highlight the role of particle size reduction on different magnetic phases.
2 Experimental details
Nanoparticles of Pr0.6-xBixSr0.4MnO3 (x = 0.10 and 0.25, for abbreviation the samples will be labelled as PB10SMO, PB25SMO respectively). were achieved using top-down approach in a two-step pathway. The methodology is pictorially represented using flowchart displayed as Fig. 1. Initially, polycrystalline bulk samples were prepared through caramic route. Highly pure Pr6O11 (99.9% trace metal basis, Powder, Sigma-Aldrich), Bi2O3 (99.9% trace metal basis, Powder, 10 μm, Sigma-Aldrich), SrCO3 (≥ 99.9% trace metal basis, Powder, Sigma-Aldrich), and MnO2 (99.9% metal basis, powder, 325 Mesh Powder, Alfa Aesar) were weighed in stoichiometric 1:1 ratio and thoroughly mixed using an agate mortar and pestle in a wet medium containing Iso-propyl alcohol. The obtained mixture was dried and then calcined in the temperature range 800–1000 ℃ for 48 h each, for 3 cycles. The mixtrure so obtained was pressed into rectangular pellets and sintered at 1100 ℃ for 24 h. Upon structural analysis and phase formation confirmation using XRD, the second step was employed. The pellets obtained from the first step were crushed into a fine powder and were subjected to high energy planetary ball milling to prepare nanoparticles. Tungsten carbide jar of 50 ml volume along with 10 mm tungsten carbide balls were used for ball milling. The sample mass was taken in such a way that the ball mass to sample mass ratio is 20:1. Ball milling was performed at the main disk speed of 300 rpm. The milling time (tm) was varied from 0 to 240 min and a small quantity of sample was taken out at regular interval of time.
To indintify the crystal structure and to evaluvate the phase purity of obtained nanoparticles, the X-ray diffraction (XRD) patterns at room temperature (RT) were taken using Bruker D2 Phaser powder X-ray diffractometer equipped Cu-Kα radiation source and LYNXEYE XE-T detector. The data was read in the 2θ range of 20–80° with a step size of 0.02°. The FullProf Suite software was used to perform Rietveld, profile matching and structure matching. The initial parameters such as space group and lattice parameters were obtained from crystallography open database (cif file) and Le bail fitting (profile matching) was performed to verify the space group and secondary phases if any. The outcomes of Le bail fitting was used for structure matching. The use of instumetal resolution parameters along with structure matching enables one to estimate the particle size and strain the structure. The microscopy measuremnts were caried out using a ZEISS Ultra-55 field emission scaning electron microscope coupled with material spectroscopy tool. The Magnetic measurements were carried out on a Quantum Design, Superconducting Quantum Interference Device (SQUID) based Vibrating Sample Magnetometer (VSM) in magnetic fields up to ± 70 kOe and in the temperature range 2–380 K. To avoid any demagnetization effects the samples were warmred above their curie point and cooled down slowly in zero field for M–H measurements.
3 Results and discussions
3.1 Room temperature X-ray diffraction and morphology
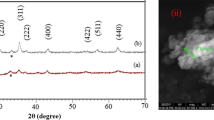
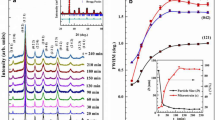
Figure 2a, b shows the RT XRD patterns of PB10SMO and PB25SMO subjected to different milling times (tm). An evident feature of the patterns upon milling is broadening of each of peak and weakening of their intensities with increase in milling time. This signifies the lack of homogeneity and lattice imperfections in the specimen. This is a common attribute of nanosized samples and hence the evidence for effective reduction in the particle size. To confirm the particle size reduction, estimation of size is inevitable. The Full Prof Suite along with instrumental resolutions is used to refine each XRD pattern and to obtain the particle size [31]. Inset in Fig. 2a, b shows the Rietveld refinement of the XRD pattern for t = 240 min ball milled nanoparticles. All the peaks in the XRD pattern of bulk, as well as ball milled nanoparticles of PB10SMO, were indexed considering the orthorhombic structure with Pnma space group whereas, the bulk PB25SMO sample showed structural polymorphism having new set of reflections in addition to those expected for orthorhombic, Pnma space group. The new set of Bragg peaks were accounted by another orthorhombic structure with Imma space group. The polymorphism is expected to exist even in nano particles. Thus, the bulk and ball milled nanoparticles of PB25SMO were analysed using two-phase Rietveld refinement considering the contribution from orthorhombic, Pnma (major phase), and Imma (minor phase) space group. A good agreement between the observed and calculated patterns and the absence of unindexed peaks rules out the possible contamination of the sample during ball milling. The unit cell parameters and the reliability values of Rietveld refinement are summarized in Table 1a, b.
The estimation of particle size (D) and micro-strain (\(\varepsilon\)) follow a set of sequences than the regular refinement [32]. Initially, the instrument resolution file (IRF) was obtained by measuring the XRD of a NIST standard sample (corundum). This file will contain the information on peak position and half widths (FWHM) of Gaussian and Lorentzian contributions of standard sample. For subsequent PBSMO samples, only the parameters influenced by the sample properties were refined. On supplying the IRF file during Rietveld refinement, Full Prof generates a microstructure file (*.mic file) where volume average of particle size (D) and micro strain (ε) would have been estimated through the integral breadth method. Figure 3a, b shows the variation in D and ε as a function of tm. A substantial decrease in D has been noticed in the initial phase of ball milling i.e. tm = 60 min in case of PB10SMO and 30 min in case of PB25SMO, thereafter D appears to level off. The ε display a monotonous increase in case of PB10SMO whereas in case of PB25SMO, ε increases up to 150 min of ball milling and then tend to saturate at higher tm. A tremendous variation in D in the early phase of ball milling could be attributed to the growth of dislocation densities (δ) and defects produced due to the collision of the balls and sample mass with walls of the jars. From D, δ can be estimated using the relation [33], \(\delta = \frac{1}{{D}^{2}}\). Inset in Fig. 3a, b shows the variation of δ with tm. The \(\delta\) value shows a substantial increase from 1013 to 1015 lines/m2 as tm changes from 0–240 min. In addition, since the feed size of the sample before ball milling is large in dimension compared to after each milling time, sizeable variation in D has been witnessed in the early phase of ball milling compared to higher tm. Thus, based on the above estimation four samples having significant variations in particle size and broad spectrum of size distribution viz. tm = 0-, 20, 120 and 240-min having D = 215, 55, 16, and 11 nm in case of PB10SMO and D = 362, 46, 21 and 16 nm in case of PB25SMO were chosen for further investigations.
Apart from X ray diffraction, transmission electron microscopy (TEM), surface area analysis which is Brunauer, Emmett, and teller method (BET) and photo correlation spectroscopy (PCS) are few of the methods to quantify the particle size of nano specimens [34] However, when the sample is magnetic TEM poses a serious limitation. In TEM the images are formed using a system of magnetic lenses. The objective lens which plays an important role in focusing and magnifying the images generates the field of the order of 2.2 T[35]. This field could easily saturate or distort the magnetic structure of sample immersed in objective lens or it can easily deflect the sample from the sample holder, leading to an eventual contamination of the lens environment, compromising its capacity. The BET method employs N2 physisorption where the sample to be characterised shall have spherical nano particles, well separated and independent of any agglomeration. Same way the PCS also require former condition to be satisfied by the nanoparticles, failing to which the and size artifact will come into picture implying the size of the agglomeration as size of the nanoparticles. To verify the state of nano particles, the extent of agglomeration and possible contamination the scanning electron microscopy (FESEM) and elemental analysis using energy-dispersive X-ray (EDS) were employed.
Figure 4 displays FESEM micrographs of all the selected specimens coded as X0 to X240 and Y0 to Y240 to differentiate between PB10SMO and PB25SMO nano particles as function of milling time. The Bulk samples in both cases have grains roughly of hexagonal shape. The grain size varies from 0.84 µm to 0.76 µm. for Practical purposes Bi2O3 is considered as efficient sintering aid as it promotes grain growth due to its liquid phase at high temperature (820 ℃). The high diffusivity of Bi2O3 improves overall diffusion of heavy ions such as Pr3+, Sr2+ present in the host matrix. But the grain growth is not a linear function of Bi2O3 concentration. The activity of Bi2O3 at high temperature in deciding the grain growth dynamics can be broadly classified into three categories depending upon the amount of liquid phase present in the specimen. (1) The bulk diffusion, where liquid phase is absent or insufficient. (2) Solution reprecipitation, where thin layer of Bi2O3 rich liquid phase is present at the grain boundaries. (3) Diffusion of atoms through excessive amount of Bi2O3 rich liquid phase. The first case leads to a non-uniform grain distribution with irregular, small grains and open porosity. This is typical of many parent compounds with insufficient sintering temperature. The second case leads uniform grain distribution with largest of all the grain in a continuous series. At this concentration the liquid Bi2O3 not only promotes the mass transfer but also enhances the wettability, leading to a rapid grain growth. The third case is where the grains dissolve or grow depending on the thickness of the wetting liquid. In the present investigation the concentration of Bi2O3 is at intermediaste stage of case 2 and 3. Which means the grain growth has been to a maximum limit and is declining after a critical concentration [36, 37]. With tm = 20 min a clear fragmentation in the grain can be seen which is result of milling process. tm = 120 min and 240 min, a clear distribution of nano particles with obvious agglomeration is seen. The grain size is estimated to be 58.9 nm, 53.8 nm, 78.2 nm and 55.3 nm respectively for PB10SMO and PB25SMO at tm = 120 min and 240 min. The disagreement in particle size estimation through X-ray diffraction and electron micrography is due to the agglomeration of nano particle leading and limited resolution of FESEM in distinguishing agglomeration and individual grains. However, the trend of particle size reduction is in concordance with the X-ray diffraction measurement. One of the major concerns of preparing the nanoparticles using top-down approach using high energy ball mill is reaction between the specimen, jar and balls. In in present case the jar and ball material was tungsten carbide. The EDS spectra was taken for each sample at least with two trails at different areas with sample area ranging from 20 µm to 90 µm. The tungsten was absent in the EDS spectra indicating the purity of preprared nano specimen. Further, the atomic percentage was expected to be Pr = 10, Bi = 2, Sr = 8, Mn = 20 and O = 60 in case of PBSMO10 and Pr = 7, Bi = 5, Sr = 8, Mn = 20 and O = 60 in case of PBSMO25. The observed atomic percentage of each element is in good agreement with expected values (The tables, spectra, and images are provided in the supplementary file).
3.2 Magnetization studies
Figure 5a, b shows the dc magnetization plots recorded in the temperature range of 3–380 K in an applied field of 100 Oe under zero field cooled (ZFC) and field cooled (FC) protocols. On lowering the temperature from 380 K, bulk (D = 215 nm) PB10SMO (Fig. 5a) undergoes a PM to FM transition at TC = 290 K, where as same state change is observed at 252 K for bulk PB25SMO (D = 362 nm) (Fig. 5b). In comparison to PSMO, which has TC at 309 K [22, 24], Bi3+ substitution for Pr3+ is found to lowers the TC which can be understood in terms of unit cell expansion. As can be seen in the structural analysis of Pr0.6-xBixSr0.4MnO3 [24, 30], the unit cell volume increases from 229.214 to 231.26 Å3 as x changes from 0 to 0.25. This, in turn, lengthens the Mn–O–Mn chain reducing the hopping probability of eg electron thereby resulting in the decrease of TC. Also, the highly covalent 6 s lone pair character of Bi3+ is expected to create localization of electrons around the Bi3+ rich region which leads to AFM coupling, diluting the FM character of PSMO [38]. Along side the Bi induced reduction TC, the low-temperature structural transition which is signature of PSMO, seen as a drop in ZFC/FC curve at TS = 88 K [22, 24] is found to get suppressed with Bi3+ substitution. The unmilled PB10SMO sample display a low temperature hump in FC at T < 200 K. Similar behaviour has been previously noticed in case of Pr0.4Bi0.2Sr0.4MnO3 [39]and La0.7-xBixSr0.3MnO3 (x = 0.30 and 0.35) [40, 41] and have been assigned to the disordered short range AFM interactions in the system. In addition, bulk PB25SMO (D = 362 nm) in addition to TC shows a kink in dM/dT at \({T}_{C}{\prime}\) = 306 K. Since the structural analysis for this system shows a phase coexistence corresponding to Pnma and Imma symmetry, the observed characteristic could be a consequence of structural phase separation in the system.
dc magnetization vs. temperature for bulk and ball milled a PB10SMO, b PB25SMO. Inset in (i) in (a) shows the variation in TC and FWHM of dM/dT vs. D−1. Inset (ii) in (a) shows the enlarged view of M–T to highlight the low temperature hump noticed. Inset in (d) shows the variation in magnetic ordering temperature with D−1
Though upon size reduction, the structural and compositional changes in the nano specimen are dormant, A dramatic reduction TC is witnessed. With a decrease in D, TC of PB10SMO (Fig. 5a) drops from 290 K (D = 215 nm) to 257 K (D = 10 nm). The low temperature hump noticed for the bulk specimen is suppressed in D = 55 nm sample and is completely absent for D = 16 and 10 nm. In case of PB25SMO, \({T}_{C}{\prime}\) decreases from 306 in bulk speciem to 295 K for D = 21 nm and is completely suppressed for D = 15 nm. The TC decreases from 252 to 203 K for D = 15 nm. In addition, the bulk (D = 362 nm) PB25SMO shows a maximum in ZFC at TM ≈ 182 K, which decreases to 109 K for D = 15 nm. Table 2 summarizes the magnetic ordering temperature corresponding to different D. To quantify these changes numerically, the percentage change in TC, \({T}_{C}{\prime}\) and TM can be considered. In case of PB10SMO ΔTC/TC% = 11.4, and with respect to PB25SMO ΔTC/TC% = 19.44, Δ \({T}_{C}{\prime}\)/\({T}_{C}{\prime}\)% = 3.6 and ΔTM/TM% = 40.1. The multiple magnetic transitions in PB25SMO perhaps find the origin vivid contribution from Ions at A-site. PB25SMO represents a system where A-site has a considerable contribution from both Pr3+ (35%) and Bi3+ (25%), multiple magnetic transitions observed suggest the presence of magnetic phase coexistence in the system. The decrease in magnetic ordering temperature with D can be explained by considering the surface effects. Since with a decrease in D, the surface effects dominate, a weak magnetic interaction among the spins on the surface of the nanoparticles results in the lowering of TC and/or TM along with broadening of magnetic transition (inset (i) in Fig. 5 a, b). Further, all samples show a large bifurcation between the ZFC and FC curves specifying the presence of inhomogeneity in the system.
Figure 6a, b shows the evolution of inverse magnetic susceptibility, \({\chi }^{-1}\) with changing temperature. The \({\chi }^{-1}(T)\) plots in the vicinity of RT (> 300 K) have been analysed adopting the Curie–Weiss law given as [42] \(\chi =\frac{C}{T-{\theta }_{p}}\); where C corresponds to the Curie constant and θp is the Curie – Weiss temperature. The solid line in the high temperature region (330–380 K) represents the linear fit given in accordance with CW law. From the slope of the linear fit, θp values are estimated. Inset in Fig. 6a, b shows the variation of θp with D−1. In all investigated specimens, θp is found to be positive. The positive value of θp signifies dominant FM interaction in both the studied system while a systematic decrease in θp with D−1 suggest a decline in the FM character of the system with a decrease in D [42]. Also from C, values of the effective paramagnetic moment (\({\upmu }_{\mathrm{eff}}\)) are estimated (tabulated in Table 2). Assuming the \({\upmu }_{\mathrm{eff}}\) values of Mn3+ as 4.90\({\upmu }_{\mathrm{B}}/\mathrm{f}.\mathrm{u}.\), Mn4+ as 3.87 \({\upmu }_{\mathrm{B}}/\mathrm{f}.\mathrm{u}.\) and Pr3+ as 3.5\({\upmu }_{\mathrm{B}}/\mathrm{f}.\mathrm{u}.\), \({\upmu }_{\mathrm{eff}}^{\mathrm{th}}\) (Theoretical value) of Pr0.6-xBixSr0.4MnO3 for x = 0.10 and 0.25 can be evaluated as,
The estimated \({\upmu }_{\mathrm{eff}}^{\mathrm{th}}\) is found to be 5.15 \({\upmu }_{\mathrm{B}}/\mathrm{f}.\mathrm{u}.\) for x = 0.10 (i.e. PB10SMO) and 4.96 \({\upmu }_{\mathrm{B}}/\mathrm{f}.\mathrm{u}.\) for x = 0.25 (i.e. PB25SMO) respectively. The experimentally determined value is higher compared to the theoretical estimation suggesting the presence of FM correlations in the PM region.
In addition, all samples show a significant deviation from the CW behaviour at temperature marked by an arrow (Fig. 7a, b). Clearly a downturn in \({\chi }^{-1}(T)\) has been noticed which emphasizes the presence of short-range FM correlations in the system. Most reports assign this anomaly to be a Griffith’s phase (GP) such as singularity in the system [43,44,45,46] while some studies consider it to be a consequence of FM polarons existing in the system above TC [47, 48].
The presence of GP like singularity in a system is characterized by a sharp downturn in \({\chi }^{-1}(T)\) for the temperature range TC ≤ T ≤ TG at a lower applied field which softens with an increase in the magnitude of external magnetic field. Also, the low field \({\chi }^{-1}\) shows a power-law dependence on temperature given as [43, 45],
where \({T}_{C}^{R}\) is the critical temperature of a random ferromagnet and the exponent \(\lambda\) determines the deviation from CW law. The temperature TG at which \({\chi }^{-1}(T)\) deviates from linearity is known as Griffith’s temperature, TG. As seen from Fig. 7a, b, the \({\chi }^{-1}(T)\) downturn which was sharp for H = 100 Oe, subdue in the presence of H = 50 kOe field proposing the applicability of GP like singularity. The suppression in \(\chi\)−1(T) downturn with H could be described as in higher external field, the magnetic contribution from PM matrix enhances and dominates the embedded FM clusters, hence driving towards linear variation in \(\chi\)−1 vs. T [49]. Also, since both \({T}_{C}^{R}\) and \(\lambda\) are variants in Eq. 2, for precise estimation of both these parameters, the procedure described in Ref. [43] has been applied. Inset in each panel of Fig. 6a, b shows the plot of \(\chi\)−1 vs. [(T/ \({T}_{C}^{R}\))–1] in double logarithmic scale. The slope of the linear fit gives the value of \(\lambda\) (tabulated in Table 2). The obtained values of \(\lambda\) are in good agreement with the reported literature [43,44,45,46] on manganite recommending the GP like singularity approach to explain deviation in \({\upchi }^{-1}(\mathrm{T})\).
The occurrence of GP like singularity in manganite is understood to be a consequence of quenched disorder due to chemical substitution [43] and grain boundary effect [45] because of particle size reduction that leads to the distribution in exchange energies resulting in the short-range FM interactions in the PM regime. Generally, in manganites A-site substitution that results in the structural distortion, competitive coexistence of AFM and FM interactions, and the magnetic interaction among different magnetic ions due to B-site substitution is considered to be a cause of the quenched disorder. In the present study, the highly covalent 6 s lone pair character of Bi3+, which results in the tilting of MnO6 octahedra along with electron localization that results in the coexistence of FM and AFM interactions is assumed to be the source of quenched disorder in bulk PBSMO. With decreasing D, in addition to the above, the broken Mn – O–Mn network, the defects induced, lattice strain, and random arrangement of the spins on the nanoparticle surface is expected to create FM fluctuations resulting in the formation of short range FM domains embedded in the PM matrix.
To get a better understanding on the magnetic state of the system upon size reduction, field dependent magnetization isotherms were recorded at selected temperatures as shown in Fig. 8a, b. In case of PB10SMO (Fig. 8a), the M–H loops at 300 K for all samples are non-linear suggesting weak FM interactions since samples are at the verge of PM to FM ordering. At T ≤ 200 K, the magnetization response of D = 215 and 55 nm is saturated with significant spontaneous moment (M0) specifying the FM ordered state of the system. However, at T ≤ 50 K, the M–H behaviour shows a slight difference between the virgin curve and the envelope (seen in the inset of Fig. 8a) suggesting a weak contribution of AFM interactions at low temperature. The observed response is in good accordance with the low-temperature hump noticed in the FC curve at T < 100 K which has been attributed to the disordered AFM interactions in the system. With a decrease in D, i.e. for D = 16 and 10 nm, the M–H isotherms are unsaturated with spontaneous moment and a significant separation between the virgin curve and loop. The observed behaviour substantiates the presence of surface disorder in nanoparticles due to ball milling which induces the strain and defects in the structure.
Compared to PB10SMO, the M–H behaviour of PB25SMO (Fig. 8b) is contrasting. At 300 K, the M–H response of D = 362 and 46 nm samples demonstrate a weak FM state as the magnetization varies nonlinearly with the ramping field. However, for D = 21 and D = 15 nm, M–H isotherm represents the PM state of the system. On lowering the temperature to T < TC, i.e., 200 K, the M–H response of D = 362 and 46 nm samples shows a significant spontaneous moment with unsaturated magnetization suggesting a FM transition in the system. At T ≤ 100 K, M–H curves are open loops along the forward and reverse field sweep with a clear step in magnetization at critical field marked as HCR. The magnetization in the virgin loop (i.e. 0–70 kOe) at 100 K and 50 K, is unsaturated with a spontaneous moment and step like feature at HCR (marked by filled arrow). In the reverse field (i.e. 70–0 kOe), the magnetization remains in the higher state (i.e. saturated state) compared to the virgin curve and then drops to zero at H = 0 kOe. Such magnetization isotherms with ramping field are regarded as metamagnetic magnetization curves which indicate field induced AFM to FM transition in the system. The spontaneous magnetization noted specify the alignment of residual FM domains parallel to the applied field. At T = 3 K, the net magnetization is lower compared to that at 100 and 50 K, which indicates overall growth in the AFM phase fraction. Also, the critical field for step, HCR is found to be large compared to the same at 100 K and 50 K, i.e. HCR ≈ 45 kOe for D = 362 nm (marked by open arrow) which increases to ≈ 50 kOe for D = 46 nm, indicates the need of higher magnetic field to bring out the AFM to FM transition in the system. With a decrease in D, i.e. D = 21 and 15 nm, the M–H loops are highly unsaturated with shrinkage in the difference between the virgin and loop curve which was clearly seen in the bulk sample. This either implies the increase in the strength of AFM interaction or a surge in the surface disorder due to ball milling that brings down the strength of FM interaction in the nanoparticles. Also, the net magnetization at 5 K which was lower compared to 100 K and 50 K for bulk and partially ball milled samples, show a systematic increase with a decrease in temperature.
Figure 9 shows the temperature evolution of saturation magnetization (MS) for PB10SMO and PB25SMO. On decreasing the temperature from 300 K, MS manifests an increase ratifying the PM to FM transition in the system. For PB10SMO, MS values saturate at T ≤ 100 K demonstrate the dominant FM state of the system. Whereas in case of PB25SMO, a drop in the value of MS has been witnessed at 3 K compared to 50 K which signifies the growth of AFM phase in the system. Figure 10 shows the variation of MS with D for PB10SMO and PB25SMO. With a decrease in D, a sizeable reduction in the magnitude of MS has been noticed. The MS value decline from 3.3 μB/f.u. to 1 μB/f.u. for PB10SMO and 2.24μB/f.u. to 0.4 μB/f.u. for PB25SMO. As a function of D−1 (inset in Fig. 10), MS displays a linear variation illustrating a strong impact of size reduction on the magnetization of the system.
Further to establish the influence of size reduction on the range of FM interactions, M2 vs. H/M (i.e., Arrott’s plots) were made (Fig. 11). If the linear intercept of the high field region of M2 vs. H/M plot yields a positive value, it indicates long range FM order in the system while the negative value represents the short range FM correlations in the system [43, 50]. As seen from Fig. 10, the intercept for PB10SMO, D = 215, 55, 16 nm is negative at T = 300 K > TC and positive at T ≤ 200 K < TC. Whereas for D = 10 nm, the intercept is negative for T ≥ 200 K and positive for T ≤ 100 K. This indicates a transition in the range of magnetic interaction from long range (T ≤ 100 K < TC) to short range (T ≥ 200 K).
In case of PB25SMO, the intercept for D = 362 and 46 nm is negative at T ≥ 200 K and positive for T ≤ 100 K indicating a transition in the range of magnetic interaction from long range to short range. Whereas in case of ball milled D = 21 and 15 nm samples the intercept is negative at all temperatures. This establishes short range of FM interactions in the system due to enhanced surface disorder.
The above results clearly manifest a strong influence of D on the magnetism of PB10SMO and PB25SMO. An increase in tm results in the decrease of D, while \(\varepsilon\) and δ show an increase. Correspondingly the TC, MS decreases while the nature of magnetic interaction changes from long range to short range. Also, the degree of unsaturation in M–H loops increases with a decrease in D. These imply a reduced magnetic interaction among the nanoparticles which can be understood in the framework of core–shell model for the nanoparticles [13, 51]. According to core–shell model, each manganite nanoparticle has a FM/AFM core surrounded by a non magnetic shell made up of broken bonds and randomly oriented magnetic spins. The existence of a shell surrounding the core has been experimentally established from TEM studies [40]. Since the magnetic spins in the shell are randomly oriented, it is considered that the shell possesses null net magnetic moment. Thus, using this assumption, we can calculate the shell thickness from the relation [13, 51],
where D is the particle size, MS (Nano), MS (Bulk) corresponds to the saturation magnetization of nanoparticles and bulk respectively. Figure 12 shows the t/D vs. D−1 plot for PB10SMO and PB25SMO. The t/D increases with a decrease in D suggest a direct impact of ball milling on the shell thickness which strongly controls the magnetic properties of the nanoparticles. However, the noted metamagnetic M–H response in bulk and partially ball milled PB25SMO samples indicates it to be the interaction among the core of the nanoparticles. Thus top-down approach using high energy ball milling serves as an interesting technique that can be used to obtain nanoparticles with bulk like properties using a cheap and without any hazardous chemicals or harmful evolution of gasses.
4 Conclusions
The effect of particle size reduction on the structure and magnetism of Pr0.6-xBixSr0.4MnO3 (x = 0.10 and 0.25) manganite nanoparticles prepared using top-down approach in a high energy planetary ball mill has been studied in detail. Conclusively, on increasing the milling time from 0 to 240 min, a dramatic change in particle size is observed, which alters the magnetism without changing bulk composition or any contaminations. The TC is a function of particle size which means there exist a freedom to tune TC to a desired point on the temperature scale without compromise in bulk properties. A Griffith’s phase like singularity whose visibility escalates upon particle size reduction is due to the quenched disorders. This causes a broad distribution of magnetic interaction on the temperature axis, hinting the extension of physical properties such as magneto resistance, magnetic entropy over a large temperature span. Size reduction supresses ferromagnetic nature and competing ferromagnetic-antiferromagnetic nature charecteristics of the parent compounds, respectively. The net magnetization decreases with a decrease in particle size. This indicates the reduced magnetic interaction in nanoparticles due to the formation of a magnetic dead layer around FM/AFM core. Further the linear dependence saturation with inverse of particle size indicates easy control over magnetism of the magnetic specimen. In general, the costeffective technique like high energy ball milling can efficiently produce nanoparticle of desired composition without large change in bulk composition, parallelly producing contrasting and highly tuneable magnetic properties. The investigation can be extended to other magnetic material having complex magnetic ground state and magento structural crossover to study changes in properties as function particle size.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Yurkov, G.Y., Gubin, S.P. and Ovchenkov, E.A., 2009. Magnetic Nanocomposites Based on the Metal‐Containing (Fe, Co, Ni) Nanoparticles inside the Polyethylene Matrix. Magnetic Nanoparticles, pp.87–115.
R. Kodama, Magnetic nanoparticles. J. Magn. Magn. Mater. 200, 359–372 (1999). https://doi.org/10.1016/S0304-8853(99)00347-9
K.M. Krishnan, A.B. Pakhomov, Y. Bao, P. Blomqvist, Y. Chun, M. Gonzales, K. Griffin, X. Ji, B.K. Roberts, Nanomagnetism and spin electronics: Materials, microstructure and novel properties. J. Mater. Sci. 41, 793–815 (2006). https://doi.org/10.1007/s10853-006-6564-1
S.C.N. Tang, I.M.C. Lo, Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 47, 2613–2632 (2013). https://doi.org/10.1016/j.watres.2013.02.039
M. Rezayeenik, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, CeVO4/rGO nanocomposite: Facile hydrothermal synthesis, characterization, and electrochemical hydrogen storage. Appl. Phys. A Mater. Sci. Process. 129, 1–12 (2023). https://doi.org/10.1007/s00339-022-06325-y
K. Mahdavi, S. Zinatloo-Ajabshir, Q.A. Yousif, M. Salavati-Niasari, Enhanced photocatalytic degradation of toxic contaminants using Dy2O3-SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach. Ultrason. Sonochem. 82, 105892 (2022). https://doi.org/10.1016/j.ultsonch.2021.105892
M.H. Esfahani, S. Zinatloo-Ajabshir, H. Naji, C.A. Marjerrison, J.E. Greedan, M. Behzad, Structural characterization, phase analysis and electrochemical hydrogen storage studies on new pyrochlore SmRETi2O7 (RE = Dy, Ho, and Yb) microstructures. Ceram. Int. 49, 253–263 (2023). https://doi.org/10.1016/j.ceramint.2022.08.338
C.P. Bean, J.D. Livingston, Superparamagnetism. J. Appl. Phys. 30, S120–S129 (1959). https://doi.org/10.1063/1.2185850
N. Abid, A.M. Khan, S. Shujait, K. Chaudhary, M. Ikram, M. Imran, J. Haider, M. Khan, Q. Khan, M. Maqbool, Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 300, 102597 (2022). https://doi.org/10.1016/j.cis.2021.102597
M.B. Salamon, M. Jaime, The physics of manganites: Structure and transport. Rev. Mod. Phys. 73, 583–628 (2001). https://doi.org/10.1103/RevModPhys.73.583
Y. Tokura, Y. Tomioka, Colossal magnetoresistive manganites. J. Magn. Magn. Mater. 200(1–3), 1–23 (1999)
C.N.R. Rao, B. Raveau, Colossal magnetoresistance, charge ordering and related properties of manganese oxides (World Scientific, 1998)
T. Zhang, X.P. Wang, Q.F. Fang, X.G. Li, Magnetic and charge ordering in nanosized manganites. Appl. Phys. Rev. (2014). https://doi.org/10.1063/14895117
M.H. Phan, S. Chandra, N.S. Bingham, H. Srikanth, C.L. Zhang, S.W. Cheong, T.D. Hoang, H.D. Chinh, Collapse of charge ordering and enhancement of magnetocaloric effect in nanocrystalline La035Pr0275Ca0375MnO3. Appl. Phys. Lett. 97, 242506 (2010). https://doi.org/10.1063/1.3526380
Markovich, V., Wisniewski, A. and Szymczak, H., 2014. Magnetic properties of perovskite manganites and their modifications. In Handbook of magnetic materials (Vol. 22, pp. 1–201). Elsevier.
Kaplan, T.A. and Mahanti, S.D. eds., 2006. Physics of manganites. Springer Science & Business Media.
A.K. Pradhan, R. Bah, R.B. Konda, R. Mundle, H. Mustafa, O. Bamiduro, R.R. Rakhimov, X. Wei, D.J. Sellmyer, Synthesis and magnetic characterizations of manganite-based composite nanoparticles for biomedical applications. J. Appl. Phys. 103, 7–10 (2008). https://doi.org/10.1063/1.2829906
D.H. Manh, P.T. Phong, P.H. Nam, D.K. Tung, N.X. Phuc, I.J. Lee, Structural and magnetic study of La0. 7Sr0. 3MnO3 nanoparticles and AC magnetic heating characteristics for hyperthermia applications. Physica B 444, 94–102 (2014)
K. Knížek, Z. Jirák, E. Pollert, F. Zounová, S. Vratislav, Structure and magnetic properties of Pr1−xSrxMnO3 perovskites. J. Solid State Chem. 100, 292–300 (1992). https://doi.org/10.1016/0022-4596(92)90103-3
O. Chmaissem, B. Dabrowski, S. Kolesnik, J. Mais, J.D. Jorgensen, S. Short, Structural and magnetic phase diagrams of La1-xSrxMnO3 and Pr1-ySryMnO3. Phys. Rev. B 67, 094431 (2003). https://doi.org/10.1103/PhysRevB.67.094431
C. Martin, A. Maignan, M. Hervieu, B. Raveau, Magnetic phase diagrams of L1−xAxMnO3 manganites (L = Pr, Sm; A = Ca, Sr). Phys. Rev. B 60, 12191–12199 (1999). https://doi.org/10.1103/PhysRevB.60.12191
D.V. Maheswar Repaka, T.S. Tripathi, M. Aparnadevi, R. Mahendiran, Magnetocaloric effect and magnetothermopower in the room temperature ferromagnet Pr0.6Sr0.4MnO3. J. Appl. Phys. doi 10(1063/1), 4769876 (2012)
M.D. Daivajna, N. Kumar, V.P.S. Awana, B. Gahtori, J. Benedict Christopher, S.O. Manjunath, K.Z. Syu, Y.K. Kuo, A. Rao, Electrical, magnetic and thermal properties of Pr0.6-xBixSr0.4MnO3 manganites. J. Alloys Compd. 588, 406–412 (2014). https://doi.org/10.1016/j.jallcom.2013.11.033
A.D. Souza, M. Vagadia, M. Daivajna, Tuning magnetic and magnetocaloric properties of Pr0.6Sr0.4MnO3 through size modifications. J. Mater. Sci. Mater. Electron. 32, 14990–15002 (2021). https://doi.org/10.1007/s10854-021-06052-9
O.S. Mantytskaya, I.O. Troyanchuk, A.N. Chobot, H. Szymczak, Magnetic phase diagram of the manganites Bi1−xSrxMnO3. Low Temp. Phys. 30, 218–224 (2004). https://doi.org/10.1063/1.1645181
M. Hervieu, A. Maignan, C. Martin, N. Nguyen, B. Raveau, Double “Mn3+” stripes in Bi1-xSrxMnO3: New type of charge ordering at room temperature. Chem. Mater. 13, 1356–1363 (2001). https://doi.org/10.1021/cm000873s
I.O. Troyanchuk, Magnetic phase diagram of the Bi1-xCaxMnO3 manganites. Phys. Solid State 44, 2266 (2002). https://doi.org/10.1134/1.1529922
H. Woo, T.A. Tyson, M. Croft, J.C. Woicik, S.W. Cheong, Correlations between the magnetic and structural properties of Ca-doped BiMnO3. Phys. Rev. B Condens. Matter Mater. Phys. 63, 1344121–13441212 (2001). https://doi.org/10.1103/physrevb.63.134412
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976). https://doi.org/10.1107/S0567739476001551
A.D. Souza, M. Vagadia, S. Chakravarty, J. Sahoo, R.J. Choudhary, S. Rayaprol, M.D. Daivajna, Influence of Bi3+ substitution on the structural and magnetic properties of Pr06Sr04MnO3 ceramics. J. Magnet. Magnet. Mater. 576, 170692 (2023)
T. Roisnel, J. Rodríquez-Carvajal, WinPLOTR: A windows tool for powder diffraction pattern analysis. Mater. Sci. Forum 378–381, 118–123 (2001). https://doi.org/10.4028/www.scientific.net/MSF.378-381.118
J. Rodríquez-Carvajal, T. Roisnel, Line broadening analysis using FullProf*: Determination of microstructural properties. Mater. Sci. Forum 443–444, 123–126 (2004). https://doi.org/10.4028/www.scientific.net/MSF.443-444.123
P. Bindu, S. Thomas, Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 8, 123–134 (2014). https://doi.org/10.1007/s40094-014-0141-9
B. Akbari, M.P. Tavandashti, M. Zandrahimi, Particle size characterization of nanoparticles–a practical approach. Iran. J. Mater. Sci. Eng. 8(2), 48–56 (2011)
D.-T. Ngo, L.T. Kuhn, In situ transmission electron microscopy for magnetic nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 7, 045001 (2016). https://doi.org/10.1088/2043-6262/7/4/045001
K. Sun, Z. Lan, Z. Yu, L. Li, J. Huang, X. Zhao, Grain growth, densification and magnetic properties of NiZn ferrites with Bi 2 O 3 additive. J. Phys. D Appl. Phys. 41, 235002 (2008). https://doi.org/10.1088/0022-3727/41/23/235002
C. Liu, Z. Lan, X. Jiang, Z. Yu, K. Sun, L. Li, P. Liu, Effects of sintering temperature and Bi2O3 content on microstructure and magnetic properties of LiZn ferrites. J. Magn. Magn. Mater. 320, 1335–1339 (2008). https://doi.org/10.1016/j.jmmm.2007.10.016
R. Seshadri, N.A. Hill, Visualizing the Role of Bi 6s “Lone Pairs” in the Off-Center Distortion in Ferromagnetic BiMnO3. Chem. Mater. 13, 2892–2899 (2001). https://doi.org/10.1021/cm010090m
A.D. Souza, M. Vagadia, M.D. Daivajna, Finite-size effects on the evolution of magnetic correlations and magnetocaloric properties of Pr0.4Bi0.2Sr0.4MnO3. Appl. Phys. A Mater. Sci. Process. 127, 1–14 (2021). https://doi.org/10.1007/s00339-021-04828-8
A.D. Souza, S. Rayaprol, M.S. Murari, M.D. Daivajna, Effect of particle size on magnetic phase coexistence in nanocrystalline La0.4Bi0.3Sr0.3MnO3. J. Supercond. Nov. Magn. 34, 3319–3331 (2021). https://doi.org/10.1007/s10948-021-06013-7
A.D. Souza, S. Rayaprol, M.S. Murari, M. Daivajna, Effect of milling on structure and magnetism of nanocrystalline La07-xBixSr03MnO3 (x= 035, 040) manganites. Phys. B Condens. Matter 606, 412792 (2021)
C. Kittel, P. McEuen, Introduction to solid state physics (John Wiley & Sons, 2018)
A.K. Pramanik, A. Banerjee, Griffiths phase and its evolution with Mn-site disorder in the half-doped manganite Pr 0.5 Sr 0.5 Mn 1-y Ga y O 3 ( y=0.0, 0.025, and 0.05). Phys. Rev. B Condens. Matter Mater. Phys. 81, 1–5 (2010). https://doi.org/10.1103/PhysRevB.81.024431
V.N. Krivoruchko, M.A. Marchenko, Griffiths phase and temporal effects in phase separated manganites. J. Magn. Magn. Mater. 412, 30–41 (2016). https://doi.org/10.1016/j.jmmm.2016.02.086
A.K. Pramanik, A. Banerjee, Finite-size effect on evolution of Griffiths phase in manganite nanoparticles. J. Phys. Condens. Matter 28, 35LT02 (2016). https://doi.org/10.1088/0953-8984/28/35/35LT02
P. Tong, B. Kim, D. Kwon, T. Qian, S.-I. Lee, S.-W. Cheong, B.G. Kim, Griffiths phase and thermomagnetic irreversibility behavior in slightly electron-doped manganites Sm1-xCaxMnO3 (0.80≤x≤0.92). Phys. Rev. B 77, 184432 (2008). https://doi.org/10.1103/PhysRevB.77.184432
J.A. Souza, J.J. Neumeier, Y.K. Yu, Magnetic signatures of ferromagnetic polarons in La0.7Ca0.3MnO3: Colossal magnetoresistance is not a Griffiths singularity. Phys. Rev. B 78, 1–5 (2008). https://doi.org/10.1103/PhysRevB.78.014436
J.A. Souza, Y.K. Yu, J.J. Neumeier, H. Terashita, R.F. Jardim, Method for analyzing second-order phase transitions: Application to the ferromagnetic transition of a polaronic system. Phys. Rev. Lett. 94, 1–4 (2005). https://doi.org/10.1103/PhysRevLett.94.207209
J. Deisenhofer, D. Braak, H.A. Krug Von Nidda, J. Hemberger, R.M. Eremina, V.A. Ivanshin, A.M. Balbashov, G. Jug, A. Loidl, T. Kimura, Y. Tokura, Observation of a griffiths phase in paramagnetic La1-xSrxMnO3. Phys. Rev. Lett. 95, 2–5 (2005). https://doi.org/10.1103/PhysRevLett.95.257202
A. Shahee, K. Singh, R.J. Choudhary, N.P. Lalla, Evidence of ferromagnetic short-range correlations in cubic La1-xSrxMnO3-δ(x=0.80, 0.85) above antiferromagnetic ordering. Phys. Status Solidi Basic Res. 252, 1832–1838 (2015). https://doi.org/10.1002/pssb.201451552
J. Curiale, R.D. Sánchez, H.E. Troiani, C.A. Ramos, H. Pastoriza, A.G. Leyva, P. Levy, Magnetism of manganite nanotubes constituted by assembled nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 75, 1–9 (2007). https://doi.org/10.1103/PhysRevB.75.224410
Acknowledgements
A.D is indebted to the Department of Science and Technology, India for financial support through the INSPIRE Fellowship (IF 170553). M. V. acknowledges the Department of Science and Technology, India for the INSPIRE faculty award (DST/INSPIRE/04/2017/003059). J.S. acknowledges the Department of Science and Technology for INSPIRE Fellowship (DST/INSPIRE/03/2018/000699). AD and MD are thankful M. Venugopal (UGC-DAE-CSR, Mumbai) for help in high energy planetary ball-milling and XRD measurements.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This work was supported by UGC-DAE-CSR, Mumbai and Department of Science and Technology, India. (Grant numbers: UDCSR/MUM/CD/CRS-M-240/2017–1008 and IF 170553) Author M.V has received research support from Department of Science and Technology, India under INSPIRE faculty award (DST/INSPIRE/04/2017/003059). Author J.S has received research support from Department of Science and Technology under INSPIRE Fellowship (DST/INSPIRE/03/2018/000699).
Author information
Authors and Affiliations
Contributions
Conceptualization, Formal Analysis, Investigation, Writing—Original Draft: ADS, Validation, Resources, Writing—Review & Editing: MV, Resources, Investigation, Data Curation: JS, Validation, Resources, Visualization, Writing—Review & Editing, Supervision: SR, Review & Editing: LDM, Supervision, Writing—Review & Editing: MD.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Souza, A.D., Sahoo, J., Vagadia, M. et al. Particle size-dependent structural and magnetic properties of Pr0.6-xBixSr0.4MnO3 (x = 0.10 and 0.25). J Mater Sci: Mater Electron 34, 1901 (2023). https://doi.org/10.1007/s10854-023-11265-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11265-1