Abstract
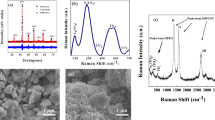
Sodium-ion batteries are being explored as an alternative to the Li-ion batteries, due to the abundance of Na and similar electrochemistry with that of Li. In this study, we report the electrochemical activity of octahedron-like antimony trioxide nanostructures for Na-ion batteries, prepared with the simple hydrothermal oxidation of antimony precursor in alkaline condition. The microstructure reveals the formation of octahedron-like microcrystals with cubic antimony trioxide phase. In Na-ion cells, the antimony trioxide electrode exhibits a reversible specific capacity of 623 mAh g−1 on the first charge and long cycle stability of 200 cycles losing only 9% capacity. The exceptional electrochemical performance achieved by antimony trioxide is owing to the conversion and alloying reactions mechanism, which accelerates the kinetics of the reactions by stabilizing the structure of anode material.





Similar content being viewed by others
References
J.-Y. Hwang, S.-T. Myung, Y.-K. Sun, Chem. Soc. Rev. 46, 3529 (2017). https://doi.org/10.1039/C6CS00776G
V. Palomares, P. Serras, I. Villaluenga, K.B. Hueso, J. Carretero-González, T. Rojo, Energy Environ. Sci. 5, 5884 (2012). https://doi.org/10.1039/C2EE02781J
V.L. Chevrier, G. Ceder, J. Electrochem. Soc. 158, A1011 (2011). https://doi.org/10.1149/1.3607983
S.W. Kim, D.H. Seo, X. Ma, G. Ceder, K. Kang, Adv. Energy Mater. 2, 710 (2012). https://doi.org/10.1002/aenm.201200026
M.M. Doeff, Y. Ma, S.J. Visco, L.C. De Jonghe, J. Electrochem. Soc. 140, L169 (1993). https://doi.org/10.1149/1.2221153
B. Xiao, T. Rojo, X. Li, ChemSusChem 12, 133 (2019). https://doi.org/10.1002/cssc.201801879
W. Xiao, Q. Sun, J. Liu et al., Nano Energy 66, 104177 (2019). https://doi.org/10.1016/j.nanoen.2019.104177
X. Ma, S. Liu, K. Zhang et al., J. Mater. Sci. Mater. Electron. 29, 3492 (2018). https://doi.org/10.1007/s10854-017-8283-6
P. Wang, X. Lu, Y. Boyjoo et al., J. Power Sources 451, 227756 (2020). https://doi.org/10.1016/j.jpowsour.2020.227756
Y. Lu, L. Yu, X.W. Lou, Chem 4, 972 (2018). https://doi.org/10.1016/j.chempr.2018.01.003
S. Ni, P. Huang, D. Chao et al., Adv. Funct. Mater. 27, 1701808 (2017). https://doi.org/10.1002/adfm.201701808
S. Ni, Q. Chen, J. Liu et al., J. Power Sources 433, 126681 (2019). https://doi.org/10.1016/j.jpowsour.2019.05.087
J. He, Y. Wei, T. Zhai, H. Li, Mater. Chem. Front. 2, 437 (2018). https://doi.org/10.1039/C7QM00480J
C. Nithya, S. Gopukumar, J. Mater. Chem. A 2, 10516 (2014). https://doi.org/10.1039/C4TA01324G
L. Wu, X. Hu, J. Qian et al., Energy Environ. Sci. 7, 323 (2014). https://doi.org/10.1039/C3EE42944J
X. Zhou, X. Liu, Y. Xu, Y. Liu, Z. Dai, J. Bao, J. Phys. Chem. C 118, 23527 (2014). https://doi.org/10.1021/jp507116t
Y. Zhu, X. Han, Y. Xu et al., ACS Nano 7, 6378 (2013). https://doi.org/10.1021/nn4025674
A. Darwiche, C. Marino, M.T. Sougrati, B. Fraisse, L. Stievano, L. Monconduit, J. Am. Chem. Soc. 134, 20805 (2012). https://doi.org/10.1021/ja310347x
B. Farbod, K. Cui, W.P. Kalisvaart et al., ACS Nano 8, 4415 (2014). https://doi.org/10.1021/nn4063598
D.-H. Nam, K.-S. Hong, S.-J. Lim, H.-S. Kwon, J. Power Sources 247, 423 (2014). https://doi.org/10.1016/j.jpowsour.2013.08.095
A. Darwiche, M.T. Sougrati, B. Fraisse, L. Stievano, L. Monconduit, Electrochem. Commun. 32, 18 (2013). https://doi.org/10.1016/j.elecom.2013.03.029
N. Li, S. Liao, Y. Sun, H.W. Song, C.X. Wang, J. Mater. Chem. A 3, 5820 (2015). https://doi.org/10.1039/C4TA06825D
W. Li, K. Wang, S. Cheng, K. Jiang, J. Mater. Chem. A 5, 1160 (2017). https://doi.org/10.1039/C6TA09265A
M. Hu, Y. Jiang, W. Sun, H. Wang, C. Jin, M. Yan, ACS Appl. Mater. Interfaces 6, 19449 (2014). https://doi.org/10.1021/am505505m
K. Li, H. Liu, G. Wang, Arab. J. Sci. Eng. 39, 6589 (2014). https://doi.org/10.1007/s13369-014-1194-4
S. Liu, Z. Cai, J. Zhou, M. Zhu, A. Pan, S. Liang, J. Mater. Chem. A 5, 9169 (2017). https://doi.org/10.1039/C7TA01895A
K.-S. Hong, D.-H. Nam, S.-J. Lim, D. Sohn, T.-H. Kim, H. Kwon, ACS Appl. Mater. Interfaces 7, 17264 (2015). https://doi.org/10.1021/acsami.5b04225
D. Wang, Y. Zhou, C. Song, M. Shao, J. Cryst. Growth 311, 3948 (2009). https://doi.org/10.1016/j.jcrysgro.2009.06.020
X. Han, M. Jin, S. Xie et al., Angew. Chem. 121, 9344 (2009). https://doi.org/10.1002/ange.200903926
Z. Sui, S. Hu, H. Chen et al., J. Mater. Chem. C 5, 5451 (2017). https://doi.org/10.1039/C7TC01289F
D.H. Nam, K.S. Hong, S.J. Lim, M.J. Kim, H.S. Kwon, Small 11, 2885 (2015). https://doi.org/10.1002/smll.201500491
S. Ni, X. Lv, J. Ma, X. Yang, L. Zhang, J. Power Sources 270, 564 (2014). https://doi.org/10.1016/j.jpowsour.2014.07.137
S. Ni, B. Zheng, J. Liu et al., J. Mater. Chem. A 6, 18821 (2018). https://doi.org/10.1039/C8TA04959A
D. Li, D. Yan, J. Ma et al., Ceram. Int. 42, 15634 (2016). https://doi.org/10.1016/j.ceramint.2016.07.017
X. Guo, X. Xie, S. Choi et al., J. Mater. Chem. A 5, 12445 (2017). https://doi.org/10.1039/C7TA02689G
Z. Yi, Q. Han, X. Li, Y. Wu, Y. Cheng, L. Wang, Chem. Eng. J. 315, 101 (2017). https://doi.org/10.1016/j.cej.2017.01.020
K. Ramakrishnan, C. Nithya, B. KundolyPurushothaman, N. Kumar, S. Gopukumar, ACS Sustain. Chem. Eng. 5, 5090 (2017). https://doi.org/10.1021/acssuschemeng.7b00469
Acknowledgments
RSK would like to thank the University Grants Commission, New Delhi, for the awarding of the D.S. Kothari Post-Doctoral Fellowship (F.4-2/2006 (BSR)/PH/14-15/0132).
Funding
University Grants Commission, New Delhi (F.4-2/2006 (BSR)/PH/14-15/0132).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10854_2020_5125_MOESM1_ESM.docx
Supplementary Information: XRD, SEM, TEM, and electrochemical performance of the rod-shaped Sb2O3, Schematic representation of coin type half-cell. (DOCX 27752 KB)
Rights and permissions
About this article
Cite this article
Kalubarme, R.S., Park, CJ., Kale, B.B. et al. Highly crystalline antimony oxide octahedron: an efficient anode for sodium-ion batteries. J Mater Sci: Mater Electron 32, 3809–3818 (2021). https://doi.org/10.1007/s10854-020-05125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05125-5