Abstract
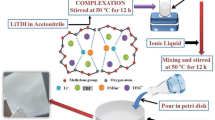
In this work, a methodical study on the influence of ionic conductivity on polymer electrolyte with different weight percentages of ionic liquid and plasticizers had been investigated in detail. Solution casting method has been employed for preparing polymer electrolyte (PE) blend having poly(vinylidenefluoride-co-hexafluoropropylene) P(VdF-co-HFP) as polymer, trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl) amide [P14,6,6,6][Tf2N] as ionic liquid and ethylene carbonate (EC) as well as propylene carbonate (PC) in 1: 1 ratio as plasticizers. The polymer electrolyte has been found out stable up to 450 °C, as measured from Thermal gravimetric analysis (TGA). Impedance spectral analysis reveals that the ionic conductivity of SPEs is 3.209 × 10–6 S/cm at 303 K with 25 wt% of ionic liquid. The addition of plasticizers (EC + PC (60 wt%)) results in two orders of magnitude of higher ionic conductivity (3.40 × 10–4 S/cm at 303 K), than that of SPEs. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscope (SEM) are performed to study the physico-chemical characteristics of polymer electrolytes. Electrochemical stability, potential window and discharge characteristics of the coin cell containing the electrolytes and LiFePO4 electrode were investigated using linear and cyclic voltammetry.









Similar content being viewed by others
References
M. Osinska, M. Walkowiak, A. Zalewska, T. Jesionowski, Study of the role of ceramic filler in composite gel electrolytes based on microporous polymer membranes. J. Membr. Sci. 326(2), 582–588 (2009). https://doi.org/10.1016/j.memsci.2008.10.036
Y. Xia, T. Fujieda, K. Tatsumi, P.P. Prosini, T. Sakai, Thermal and electrochemical stability of cathode materials in solid polymer electrolyte. J. Power Sources 92(1–2), 234–243 (2001). https://doi.org/10.1016/S0378-7753(00)00533-4
G. Li, Z. Li, P. Zhang, H. Zhang, Y. Wu, Research on a gel polymer electrolyte for Li-ion batteries. Pure Appl. Chem. 80(11), 2553–2563 (2008). https://doi.org/10.1351/pac200880112553
C.S. Kim, S.M. Oh, Importance of donor number in determining solvating ability of polymers and transport properties in gel-type polymer electrolytes. Electrochim. Acta 45(13), 2101–2109 (2000). https://doi.org/10.1016/S0013-4686(99)00426-0
F.B. Dias, L. Plomp, J.B. Veldhuis, Trends in polymer electrolytes for secondary lithium batteries. J. Power Sources 88(2), 169–191 (2000). https://doi.org/10.1016/S0378-7753(99)00529-7
O.V. Bushkova, S.E. Popov, T.V. Yaroslavtseva, V.M. Zhukovsky, A.E. Nikiforov, Ion–molecular and ion–ion interactions in solvent-free polymer electrolytes based on amorphous butadiene—acrylontrile copolymer and LiAsF6. Solid State Ion. 178(35–36), 1817–1830 (2008). https://doi.org/10.1016/j.ssi.2007.11.023
S. Ferrari, E. Quartarone, P. Mustarelli, A. Magistris, M. Fagnoni, S. Protti, C. Gerbaldi, A. Spinella, Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis (trifluoromethanesulfonyl)-imide ionic liquid. J. Power Sources 195(2), 559–566 (2010). https://doi.org/10.1016/j.jpowsour.2009.08.015
J.P. Tafur, F. Santos, A.J. Romero, Influence of the ionic liquid type on the gel polymer electrolytes properties. Membranes 5(4), 752–771 (2015). https://doi.org/10.3390/membranes5040752
D. Saikia, Y.W. Chen-Yang, Y.T. Chen, Y.K. Li, S.I. Lin, Li NMR spectroscopy and ion conduction mechanism of composite gel polymer electrolyte: A comparative study with variation of salt and plasticizer with filler. Electrochim Acta 54(4), 1218–1227 (2009). https://doi.org/10.1016/j.electacta.2008.09.001
S.K. Chaurasia, R.K. Singh, Crystallization behaviour of a polymeric membrane based on the polymer PVdF–HFP and the ionic liquid BMIMBF 4. RSC Adv. 4(92), 50914–50924 (2014). https://doi.org/10.1039/C4RA07085B
D. Saikia, Y.W. Chen-Yang, Y.T. Chen, Y.K. Li, S.I. Lin, Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P (VDF-HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 234(1–3), 24–32 (2008). https://doi.org/10.1016/j.desal.0000.00.000
L.N. Sim, S.R. Majid, A.K. Arof, Effects of 1–butyl–3–methyl imidazolium trifluoromethanesulfonate ionic liquid in poly (ethyl methacrylate)/poly (vinylidenefluoride-co-hexafluoropropylene) blend based polymer electrolyte system. Electrochim Acta 123, 190–197 (2014). https://doi.org/10.1016/j.electacta.2014.01.017
T. Michot, A. Nishimoto, M. Watanabe, Electrochemical properties of polymer gel electrolytes based on poly (vinylidene fluoride) copolymer and homopolymer. Electrochim Acta 45(8–9), 1347–1360 (2000). https://doi.org/10.1016/S0013-4686(99)00343-6
B.G. Soares, K. Pontes, J.A. Marins, L.F. Calheiros, S. Livi, G.M. Barra, Poly (vinylidene fluoride-co-hexafluoropropylene)/polyaniline blends assisted by phosphonium–based ionic liquid: dielectric properties and β-phase formation. Eur. Polym. J. 73, 65–74 (2015). https://doi.org/10.1016/j.eurpolymj.2015.10.003
R.E. Ramírez, L.C. Torres-González, E.M. Sánchez, Electrochemical aspects of asymmetric phosphonium ionic liquids. J. Electrochem. Soc. 154(2), 229–233 (2007). https://doi.org/10.1149/1.2404789
E.H. Cha, J.Y. Mun, E. Cho, T.E. Yim, Y.G. Kim, S.M. Oh, S.A. Lim, J.W. Lim, The corrosion study of al current collector in phosphonium ionic liquid as solvent for lithium ion battery. J. Korean Electrochem. Soc. 14(3), 152–156 (2011). https://doi.org/10.5229/JKES.2011.14.3.152
M. Taige, D. Hilbert, T.J. Schubert, Mixtures of ionic liquids as possible electrolytes for lithium ion batteries. Int. J. Res. Phys. Chem. Chem. Phys. 226(2), 129–139 (2012). https://doi.org/10.1524/zpch.2012.0161
H.L. WuTY, P.R. Chen, J.W. Liao, Ionic conductivity and transporting properties in LiTFSI-doped bis (trifluoromethanesulfonyl) imide-based ionic liquid electrolyte. Int. J. Electrochem. Sci. 8, 2606–2624 (2013)
S.S. Keskar, L.A. Edye, C.M. Fellows, W.O.S. Doherty, ATR-FTIR measurement of biomass components in phosphonium ionic liquids. J. Wood Chem. Technol. 32, 175–186 (2012). https://doi.org/10.1080/02773813.2011.631718
S.R. Sarda, W.N. Jadhav, A.S. Shete, K.B. Dhopte, S.M. Sadawarte, P.J. Gadge, R.P. Pawar, Phosphonium ionic liquid–catalyzed Michael addition of mercaptans to α, β-unsaturated ketones. Synth. Commun. 40(14), 2178–2184 (2010). https://doi.org/10.1080/00397910903221050
J.W. Vaughan, D. Dreisinger, J. Haggins, Density, viscosity, and conductivity of tetraalkyl phosphonium ionic liquids. ECS Trans. 2(3), 381–392 (2006). https://doi.org/10.1149/1.2196027
A.F. Ferreira, P.N. Simoes, A.G. Ferreira, Quaternary phosphonium-based ionic liquids: thermal stability and heat capacity of the liquid phase. J. Chem. Thermodyn. 45(1), 16–27 (2012). https://doi.org/10.1016/j.jct.2011.08.019
M. Nadherna, J. Reiter, J. Moskon, R. Dominko, Lithium bis (fluorosulfonyl) imide–PYR14TFSI ionic liquid electrolyte compatible with graphite. J. Power Sources 196(18), 7700–7706 (2011). https://doi.org/10.1016/j.jpowsour.2011.04.033
P.A. Thomas, B.B. Marvey, Room temperature ionic liquids as green solvent alternatives in the metathesis of oleochemical feedstocks. Molecules 21(2), 184 (2016). https://doi.org/10.3390/molecules21020184
A.J. Rennie, N. Sanchez-Ramirez, R.M. Torresi, P.J. Hall, Ether-bond-containing ionic liquids as supercapacitor electrolytes. J Phys Chem Lett 4(17), 2970–2974 (2013). https://doi.org/10.1021/jz4016553
R. Zhang, Y. Chen, R. Montazami, Ionic liquid-doped gel polymer electrolyte for flexible lithium-ion polymer batteries. Materials 8(5), 2735–2748 (2015). https://doi.org/10.3390/ma8052735
S.K. Chaurasia, R.K. Singh, S. Chandra, Thermal stability, complexing behavior, and ionic transport of polymeric gel membranes based on polymer PVdF-HFP and ionic liquid,[BMIM][BF4]. J. Phys. Chem. B 117(3), 897–906 (2013). https://doi.org/10.1021/jp307694q
P.K. Singh, K.C. Sabin, X. Chen, Ionic liquidsolid polymer electrolyte blends for supercapacitor applications. Polym. Bull. 73, 255–263 (2016). https://doi.org/10.1007/s00289-015-1484-3
M. Wang, S.A. Vail, A.E. Keirstead, M. Marquez, D. Gust, A.A. Garcia, Preparation of photochromic poly (vinylidene fluoride-co-hexafluoropropylene) fibers by electrospinning. Polymer 50(16), 3974–3980 (2009). https://doi.org/10.1016/j.polymer.2009.06.044
A.H. Battez, M. Bartolome, D. Blanco, J.L. Viesca, A. Fernandez-Gonzalez, R. Gonzalez, Phosphonium cation-based ionic liquids as neat lubricants: physicochemical and tribological performance. Tribol. Int. 95, 118–131 (2016). https://doi.org/10.1016/j.triboint.2015.11.015
M. Dobbelin, I. Azcune, M. Bedu, A. Ruiz de Luzuriaga, A. Genua, V. Jovanovski, G. Cabanero, I. Odriozola, Synthesis of pyrrolidinium-based poly (ionic liquid) electrolytes with poly (ethylene glycol) side chains. Chem. Mater. 24(9), 1583–1590 (2012). https://doi.org/10.1021/cm203790z
A.R. Polu, D.K. Kim, H.W. Rhee, Poly (ethylene oxide)-lithium difluoro (oxalato) borate new solid polymer electrolytes: ion–polymer interaction, structural, thermal, and ionic conductivity studies. Ionics 21(10), 2771–2780 (2015). https://doi.org/10.1007/s11581-015-1474-1483
F. Deng, X. Wang, D. He, J. Hu, C. Gong, Y.S. Ye, X. Xie, Z. Xue, Microporous polymer electrolyte based on PVDF/PEO star polymer blends for lithium ion batteries. J. Membr. Sci. 491, 82–89 (2015). https://doi.org/10.1016/j.memsci.2015.05.021
M. Ravi, S. Song, J. Wang, T. Wang, R. Nadimicherla, Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J. Mater. Sci.: Mater. Electron. 27(2), 1370–1377 (2016). https://doi.org/10.1007/s10854-015-3899-x
J.H. Shin, W.A. Henderson, C. Tizzani, S. Passerini, S.S. Jeong, K.W. Kim, Characterization of solvent-free polymer electrolytes consisting of ternary PEO–LiTFSI–PYR14 TFSI. J. Electrochem. Soc. 153(9), A1649-1654 (2006). https://doi.org/10.1149/1.2211928
K. Tsunashima, F. Yonekawa, M. Kikuchi, M. Sugiya, Tributylmethylphosphonium Bis (trifluoromethylsulfonyl) amide as an effective electrolyte additive for lithium secondary batteries. J. Electrochem. Soc. 157(11), A1274-1278 (2010). https://doi.org/10.1149/1.3490662
K. Xu, Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104(10), 4303–4418 (2004). https://doi.org/10.1021/cr030203g
S. Seki, Y. Ohno, Y. Mita, N. Serizawa, K. Takei, H. Miyashiro, Imidazolium-based room-temperature ionic liquid for lithium secondary batteries: relationships between lithium salt concentration and battery performance characteristics. ECS Electrochem. Lett. 1(6), A77 (2012). https://doi.org/10.1149/2.003206eel
A. Swiderska-Mocek, Electrolyte based on 1-ethyl-3-vinylimidazolium bis (trifluoromethanesulphonyl) imide for Li-ion batteries. Electrochim Acta 132, 504–511 (2014). https://doi.org/10.1016/j.electacta.2014.03.185
Acknowledgements
The author M. Sivakumar gratefully acknowledges for the financial support to carry out this work by University Grants Commission (UGC), New Delhi, Govt. India, under major research project (F.No.41-839/2012(SR)). Also, all the authors gratefully acknowledge for extending the analytical facilities in the Department of Physics, Alagappa University under the PURSE programme, sponsored by Department of Science and Technology (DST) New Delhi, Govt. of India and Ministry of Human Resource Development RUSA- Phase 2.0 Grant sanctioned vide Lt.No.F-24-51/2014 U Policy (TN Multi Gen), Dept. of Education, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muthupradeepa, R., Sivakumar, M., Subadevi, R. et al. Physical and electrochemical chattels of phosphonium ionic liquid-based solid and gel-polymer electrolyte for lithium secondary batteries. J Mater Sci: Mater Electron 31, 22933–22944 (2020). https://doi.org/10.1007/s10854-020-04820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04820-7