Abstract
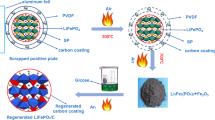
Typically, LiFePO4 batteries (LFPBs) contain a shell, cathode mixture materials, anode mixture materials, current collector, electrolyte, separator, and other components. Cathode mixture materials are composed of a binder, conductive additive, and LiFePO4/C. After LFPBs are scrapped, their appropriate disposal is necessary to avoid pollution. This study investigated the recovery of Li, Fe, and P by hydrometallurgy from scrapped LFPBs. To remove the binder, conductive additive, and carbon coating layer, recycled cathode mixture materials are oxidized to Li3Fe2(PO4)3 and Fe2O3 at 600 °C. Using H3PO4 as a leaching agent, the optimal leaching efficiencies, i.e., 99.2% and 97.68% for Li and Fe, respectively, can be achieved when the reaction time, temperature, Li in the Li3Fe2(PO4)3 and Fe2O3 mixture materials to the H3PO4 molar ratio (L/P ratio), and H3PO4 concentration are 12 h, 95 °C, 1:5, and 0.5 mol/L, respectively. Moreover, Li can be leached into a solution efficiently and recovered as LiH2PO4, while Fe and P can be selectively precipitated as FePO4·xH2O. FePO4 is prepared by a heat treatment. Furthermore, LiFePO4/C is re-synthesized by FePO4, LiH2PO4, Fe2O3, LiOH, and sucrose.






Similar content being viewed by others
References
W. Wang, Y.F. Wu, An overview of recycling and treatment of spent LiFePO4 batteries in China. Resour. Conserv. Recycl. 127, 233–243 (2017)
Y. Jiang, R.Y. Tian, H.Q. Liu, J.K. Chen, X.H. Tan, L.N. Zhang, G.Y. Liu, H.F. Wang, L.F. Sun, WeiG Chu, Synthesis and characterization of oriented linked LiFePO4 nanoparticles with fast electron and ion transport for high-power lithium-ion batteries. Nano Res. 8, , 660–665 –3814 (2015). https://doi.org/10.1038/ncb1595
L. Li, J. Ge, F. Wu, R.J. Chen, S. Chen, B.R. Wu, Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 176, 288–293 (2010)
L.G. Lu, X.B. Han, J.Q. Li, J.F. Hua, M.G. Ouyang, A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 226, 272–288 (2013)
E.A. Olivetti, G. Ceder, G.G. Gaustad, X.K. Fu, Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule 1, 229–243 (2017)
L. Zhi, Z. Peng., W. Zhifu, S. Qiang, R. Yinan, State of charge estimation for Li-ion battery based on extended kalman filter. Energy Procedia 105, 3515–3520 (2017)
K. Richa, C.W. Babbitt, N.G. Nenadic, G. Gaustad, Environmental trade-offs across cascading lithium-ion battery life cycles. Int. J. Life Cycle Assess. 22, 66–81 (2017)
K.L. Liu, K. Li, Z.L. Yang, C. Zhang, J. Deng, An advanced Lithium-ion battery optimal charging strategy based on a coupled thermoelectric model. Electrochim. Acta 225, 330–344 (2017)
G.E. Blomgren, The development and future of lithium ion batteries. J. Electrochem. Soc. 164(1), A5019–A5025 (2017)
M.T. Esfandarani, S. Rousselot, M. Gauthier, P. Sauriol, M. Duttine, A. Wattiaux, Y. Liu, A.X. Sun, G. Liang, M. Dollé, Control of the LiFePO4 electrochemical properties using low-cost iron precursor in a melt process. J. Solid State Electrochem. 20, 3481–3490 (2016)
J. Ordoñez, E.J. Gago, A. Girard, Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 60, 195–205 (2016)
C.L. Gong, Z.G. Xue, S. Wen, Y.S. Ye, X.L. Xie, Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 318, 93–112 (2016)
A. Eftekhari, Y. Liu, P. Chen, Different roles of ionic liquids in lithium batteries. J. Power Sources 334, 221–239 (2016)
W.F. Gao, X.H. Zhang, X.H. Zheng, X. Lin, H.B. Cao, Y. Zhang, Sun, lithium carbonate recovery from cathode scrap of spent lithium ion battery: a closed-loop process. Environ. Sci. Technol. 51, 1662–1669 (2017)
X.L. Li, J. Zhang, D.W. Song, J.S. Song, L.Q. Zhang, Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 345, 78–84 (2017)
X.G. Teng, F.Q. Li, P.H. Ma, Q.D. Ren, S.Y. Li,Study on thermal decomposition of lithium hexafluorophosphate by TG-FT-IR coupling method. Thermochim. Acta 436, 30–34 (2005)
K. Kanamura, H. Tamura, S. Shiraishi, Z.I. Takehare, XPS analysis for the lithium surface immersed in γ-butyrolactone containing various salts. Electrochim. Acta 40, 913–921 (1995)
D.C. Bian, Y.G. Sun, S. Li, Y. Tian, Z.H. Yang, X.M. Fan, W.X. Zhang, A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers. Electrochim. Acta 190, 134–140 (2016)
R.J. Zheng, L. Zhao, W.H. Wang, Y.L. Liu, Q.X. Ma, D.Y. Mu, R.H. Lia, C.S. Dai, Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 6, 43613–43625 (2016)
Y.X. Yang, X.H. Zheng, H.B. Cao, C.L. Zhao, X. Lin, P.G. Ning, Y. Zhang, W. Jin, Z. Sun, A closed-loop process for selective metal recovery from spent lithium iron phosphate batteries through mechanochemical activation. ACS Sustain. Chem. Eng. 5, 8017–8024 (2017)
H. Li, S.Z. Xing, Y. Liu, F.J. Li, H. Guo, G. Kuang, Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system. ACS Sustain. Chem. Eng. 5, 8017–8024 (2017)
G. Rousse, J. Rodríguez-Carvajal, C. Wurm, C. Masquelier, Magnetic structural studies of the two polymorphs of Li3Fe2(PO4)3: analysis of the magnetic ground state from super-super exchange interactions. Chem. Mater. 13, 4527–4536 (2001)
D. Morgan, G. Ceder, M.Y. Saïdi, J. Barker, J. Swoyer, H. Huang, G. Adamson, Experimental and computational study of the structure and electrochemical properties of LixM2(PO4)3 compounds with the monoclinic and rhombohedral structure. Chem. Mater. 14, 4684–4693 (2002)
A.S. Andersson, B. Kalska, P. Jönsson, L. Häggström, P. Nordblad, R. Tellgrena, J.O. Thomas, The magnetic structure and properties of rhombohedral Li3Fe2(PO4)3. J. Mater. Chem. 10, 2542–2547 (2000)
Acknowledgements
The authors acknowledge support from the Natural Science Foundation of Hunan Province (Grant No. 2017JJ2168), Guangxi Science and Technology Plan Project (Grant No. 2017GXNSFBA198187), Liuzhou Science and Technology Plan Project (Grant No. 2018DH10505).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, L., He, H. et al. Preparation of FePO4 and LiH2PO4 from cathode mixture materials of scrapped LiFePO4 batteries. J Mater Sci: Mater Electron 31, 4083–4091 (2020). https://doi.org/10.1007/s10854-020-02955-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-02955-1