Abstract
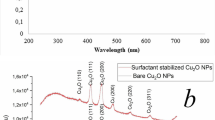
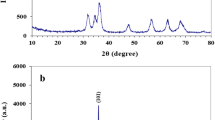
Nanostructured materials play a significant role in antibacterial activities. However, understanding the geometrical influence at the nanoscale in terms of size- and shape-correlated physical properties on antibacterial activities is very essential. Herein, we report the antibacterial influence of various copper oxide nanostructures (CuO NS) such as nanoparticles (NPs) (< 10 nm), nanospheres (NSs) (50–100 nm), and porous nanoflowers (NFs) (≈ 350 nm). The XRD confirmed the crystalline nature of CuO NPs without impurities. The antibacterial activities of CuO NPs were investigated by the microplate dilution method and confocal laser scanning microscopic (CLSM) imaging. NPs having a diameter less than 10 nm exhibited significant damage to the bacterial cell membrane than NSs and NFs. Interestingly, NPs illustrated relatively low antibacterial activity against Gram-negative bacteria (Pseudomonas aeruginosa and Acinetobacter baumannii) than Gram-positive bacteria (Staphylococcus aureus and Staphylococcus epidermidis). Acinetobacter baumannii was found to be more susceptible to the NPs than other bacterial strains, attributed to its increased membrane permeability. The death phase was observed at a concentration of 15.6 µg mL−1 and 3.9 µg mL−1 for P. aeruginosa and A. baumannii, respectively, when treated with CuO NP after the 8 h of incubation. Similarly, for S. aureus and S. epidermidis, the death phase was observed at the concentration of 31.2 µg mL−1 and 250 µg mL−1, respectively. Furthermore, as the cell cytotoxicity measurements against human fibroblast L9239 cells revealed that CuO NPs were safe. The morphological and cell viability assay demonstrated 100% cell survival, when treated with NPs and NSs (5, 10, and 25 µg mL−1), signifies no cytotoxicity. Therefore, CuO nanoparticles can be used for clinical and therapeutic applications.
Graphical Abstract















Similar content being viewed by others
References
Church NA, McKillip JL (2021) Antibiotic resistance crisis: challenges and imperatives. Biologia 76:1535–1550
Khan SN, Khan AU (2016) Breaking the spell: combating multidrug resistant “Superbugs.” Front Microbiol 7:174
Qais FA, Shafiq A, Khan HM, Husain FM, Khan RA, Alenazi B, Alsalme A, Ahmad I (2019) Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg Chem Appl 2019:4649506
Ohikhena FU, Wintola OA, Afolayan AJ (2017) Evaluation of the antibacterial and antifungal properties of Phragmanthera capitata (Sprengel) Balle (Loranthaceae), a Mistletoe growing on rubber tree, using the dilution techniques. Sci World J 2017:9658598
Ingle AP, Duran N, Rai M (2014) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98(3):1001–1009
Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192
Weinberg SE, Villedieu A, Bagdasarian N, Karah N, Teare L, Elamin WF (2020) Control and management of multidrug resistant Acinetobacter baumannii: a review of the evidence and proposal of novel approaches. Infect Prevent Pract 2(3):100077
Ruppé É, Woerther PL, Barbier F (2015) Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care 5(1):61
Parlet CP, Brown MM, Horswill AR (2019) commensal staphylococci influence staphylococcus aureus skin colonization and disease. Trends Microbiol 27:497–507
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nat Rev Microbiol 16:143–155
Chessa D, Ganau G, Spiga L, Bulla A, Mazzarello V, Campus GV, Rubino S (2016) staphylococcus aureus and staphylococcus epidermidis virulence strains as causative agents of persistent infections in breast implants. PLoS ONE 11(1):01466682016
Context L (2021) Antimicrobial resistance in nosocomial isolates of gram-negative bacteria: public health implications in the latvian context. Antibiotics 10(7):791
Khan HA, Ahmad A, Mehboob R (2015) Nosocomial infections and their control strategies, Asian Pac. J Trop Biomed 5:509–514
Reygaert WC (2018) An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 4:482–501
D’andrea MM, Fraziano M, Thaller MC, Rossolini GM (2019) The urgent need for novel antimicrobial agents and strategies to fight antibiotic resistance. Antibiotics 8:10–12
Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R (2015) Alternative antimicrobial approach: nano-antimicrobial materials. J Evid Based Complement Altern Med 2015:246012
Lee NY, Ko WC, Hsueh PR (2019) Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol 10:1153
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int J Nanomedicine 12:1227–1249
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J Nanobiotechnology 15:1–20
Richter K, Thomas N, Claeys J, McGuane J, Prestidge C, Coenye T, Wormald P, Vreugde S (2017) A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob Agents Chemother 61(6):00481–00517
Halder U, Roy R, Biswas R, Khan D, Mazumder K, Bandopadhyay R (2022) Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem Engg J Adv 11:100294
Ssekatawa K, Byarugaba DK, Angwe MK, Wampande EM, Ejobi F, Nxumalo E, Maaza M, Sackey J, Kirabira JB (2022) Phyto-Mediated Copper Oxide Nanoparticles for Antibacterial, Antioxidant and Photocatalytic Performances. Front Bioeng Biotechnol 10:820218
Shehabeldine AM, Amin BH, Hagras FA et al (2022) Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl Biochem Biotechnol 195(1):467–485. https://doi.org/10.1007/s12010-022-04120-2
Dulta K, Koşarsoy Ağçeli G, Chauhan P et al (2022) Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain Environ Res 32:2. https://doi.org/10.1186/s42834-021-00111-w
Shah IH, Ashraf M, Sabir I, Manzoor MA, Malik M, Gulzar S, Ashraf F, Iqbal J, Niu Q, Zhang Y (2022) Green synthesis and characterization of copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J Molecular Struct 1259:132696. https://doi.org/10.1016/j.molstruc.2022.132696
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79
Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C (2020) Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther 20(1):55. https://doi.org/10.1186/s12906-020-2837-5
Navale GR, Rout CS, Gohil KN, Dharne MS, Late DJ, Shinde SS (2015) Oxidative and membrane stress-mediated antibacterial activity of WS2 and rGO-WS2 nanosheets. RSC Adv 5:74726–74733
Navale GR, Thripuranthaka M, Late DJ, Shinde SS (2015) Antimicrobial Activity of ZnO Nanoparticles against Pathogenic Bacteria and Fungi. JSM Nanotechnol Nanomed 3:1033
Navale GR, Dharne MS, Shinde SS (2015) Antibiofilm activity of tert-BuOH functionalized ionic liquids with methylsulfonate counteranions. RSC Adv 5:68136–68142
Robertson J, McGoverin C, Vanholsbeeck F, Swift S (2019) Optimisation of the protocol for the LIVE/DEAD® BacLightTM bacterial viability kit for rapid determination of bacterial load. Front Microbiol 10:801
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Kielkopf CL, Bauer W, Urbatsch IL (2020) Bradford assay for determining protein concentration. Cold Spring Harb Protoc 2020(4):102269
Lin YSE, Vidic RD, Stout JE, McCartney CA, Yu VL (1998) Inactivation of Mycobacterium avium by copper and silver ions. Water Res 32:1997–2000
Cavassin ED, de Figueiredo LFP, Otoch JP, Seckler MM, de Oliveira RA, Franco FF, Marangoni VS, Zucolotto V, Levin ASS, Costa SF (2015) Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J Nanobiotechnology 13:1–16
Niño-Martínez N, Salas Orozco MF, Martínez-Castañón G-A, Torres Méndez F, Ruiz F (2019) Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int J Mol Sci 20:2808
Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR (2018) Nano-strategies to fight multidrug resistant bacteria “a battle of the titans.” Front Microbiol 9:1441
Desai DG, Swarali H, Navale GR, Prabhune A, Late DJ, Dharne MS, Walke PS (2021) Inhibition of quorum sensing, motility and biofilm formation of pseudomonas aeruginosa by copper oxide nanostructures. J Clust Sci 32(6):1531–1541
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C 44:278–284
Ahamed M, Alhadlaq H A, Majeed Khan MA, Karuppiah P, Al-Dhabi Naif A (2014) Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J Nanomater 2014:1–4. https://doi.org/10.1155/2014/637858
Khashan K S, Sulaiman G M, Abdulameer F A (2016) Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arabian J Sci Eng 41(1):301–310. https://doi.org/10.1007/s13369-015-1733-7
Ren G, Hu D, Cheng E et al (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicro Agent 33(6):587–590
Nithiyavathi R, John Sundaram S, Theophil Anand G, Raj Kumar D, Dhayal Raj A, Al Farraj DA, Aljowaie RM, AbdelGawwad MR, Samson Y, Kaviyarasu K (2021) Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J Infect Public Health 14:1893–1902
Romaniuk JAH, Cegelski L (2015) Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos Trans R Soc B Biol Sci 370:20150024
Pasquina-Lemonche L, Burns J, Turner RD, Kumar S, Tank R, Mullin N, Wilson JS, Chakrabarti B, Bullough PA, Foster SJ, Hobbs JK (2020) The architecture of the Gram-positive bacterial cell wall. Nature 582:294–297
Brown AR, Gordon RA, Hyland SN, Siegrist MS, Grimes CL (2020) Chemical biology tools for examining the bacterial cell wall. Cell Chem Biol 27:1052–1062
Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA (2012) Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett 71:114–116
Verma N, Kumar N (2019) Synthesis and biomedical applications of copper oxide nanoparticles: an expanding horizon, ACS biomater. Sci Eng 5:1170–1188
Tortella G, Rubilar O, Fincheira P, Pieretti JC, Duran P, Lourenço IM, Seabra AB (2021) Bactericidal and virucidal activities of biogenic metal-based nanoparticles: advances and perspectives. Antibiotics 10(7):783
Khalid A, Ahmad P, Alharthi AI, Muhammad S, Khandaker MU, Faruque MRI, Din IU, Alotaibi MA, Khan A (2021) Synergistic effects of Cu-doped ZnO nanoantibiotic against Gram-positive bacterial strains. PLoS ONE 16(5):e0251082
Acknowledgements
We appreciate the Director, CSIR-National Chemical Laboratory, Pune, and the Director, National Center for Nanoscience and Nanotechnology, University of Mumbai (NCNNUM), for providing infrastructure and facilities for the research work. Mr. Harishchandra Nishad and Dr. Shobhnath Gupta are acknowledged for their valuable help during characterization and discussion.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desai, D.G., Navale, G.R., Late, D.J. et al. Size does matter: antibacterial activities and cytotoxic evaluation of polymorphic CuO nanostructures. J Mater Sci 58, 2782–2800 (2023). https://doi.org/10.1007/s10853-023-08157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08157-4