Abstract
The mud crab, Scylla serrata, has huge claws in comparison with its body size. The color of the claw top’s finger surface changes from white to deep-mottled blue, and this discoloration was strongly associated with the change in hardness inside the finger cross section. With special attention to the discoloration points, the tissue structure of the exoskeleton was investigated via scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction (XRD), and the mechanical properties were examined using Vickers hardness and nanoindentation tests. The exocuticle in the deep blue surface exoskeleton was as thin as that in other crustaceans, and the exoskeleton was occupied by the endocuticle with a twisted plywood structure. On the other hand, in the white surface exoskeleton, the thickness of the hard and dense exocuticle accounted for 52–59% of the exoskeleton thickness. This percentage increased at the claw tip. The hardness of the exocuticle was 2.5 times that of the endocuticle, and the microstructures and mechanical properties gradually varied at the boundary between the exo- and endocuticle. The mechanical properties were almost constant in the exocuticle, but calcium (Ca) concentrations decreased from the outer surface toward the boundary in that region and magnesium (Mg) concentrations increased. The change in the unit cell volume obtained via XRD suggested that some of the Ca atoms in the calcite crystal structure in that region were replaced with Mg atoms. Changes in crustacean coloration may help us to understand the tissue structure and mechanical properties within the exoskeleton.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of biomimetics has been around for a long time as a technology that breaks existing conventions [1,2,3]. The excellent characteristics of organisms, such as the toughness of abalone shells [4, 5], the impact absorption of dactyl clubs hammers [6, 7], and the hard fangs of spiders [8], have been investigated through macro-, meso-, and microstructural observations and various mechanical tests. The interior of these organisms has a complicated hierarchical structure far beyond our imagination. Through cutting-edge methods of materials engineering (especially observation technology), we can learn many diverse tissue structures from organisms (biological seeds) by “becoming able to see what was invisible.” Moreover, numerical simulations such as finite element analysis reveal the mechanisms of the excellent characteristics of organisms [9, 10], and new material-processing technologies such as additive manufacturing (3D printing) can be used to create materials with these complex hierarchical structures [1, 11, 12]. Biomimetics research is expected to accelerate its expansion into the medical, automobile, and aviation industries.
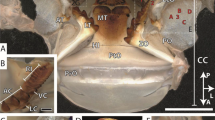
In the biological world, force (F), such as biting and pinching, has a positive correlation to body weight (BW) [13]. This correlation is reasonable because the body weight increases, the body grows, and the contact area increases. However, the maximum force per unit of body weight (F/BW) has a negative correlation to BW [14]. Crustaceans have the highest such correlation, and they have a cheliped/claw with excellent mechanical properties [15, 16]. Zang et al. [9] revealed the excellent properties and clamping behaviors of snow crab (Chionoecetes opilio) claws through mechanical testing and finite element analysis. Among crustaceans, the mud crab, Scylla serrata [17,18,19], has characteristically huge claws for crushing their staple food, bivalve mollusks, as shown in Fig. 1(a). These claws are much larger than those of the coconut crab (Birgus latro), the largest terrestrial crustacean, at the same BW [20]. However, the exoskeleton of the mud crab claws has hardly been studied as compared to the exoskeleton of claws of other decapod crustaceans: the coconut crab; American lobster, Homarus americanus; European edible crab, Cancer pagurus; Chinese mitten crab, Eriocheir sinensis; and the snapping shrimp, Alpheus heterochaelis [21,22,23,24,25,26,27]. As shown in Fig. 1a, the mud crab’s entire body is deep-mottled blue. In general, body colors vary from deep-mottled green to dark brown, depending on the habitat. This is due to the carotenoproteins, which are a combination of a pigment called astaxanthin and a protein [28]. However, a white area different from the denticle can be seen at the tip of the finger of the claws, as shown in Fig. 1b [29]. Such a feature is also found in the claws of the blue crab, Callinectes sapidus, the green crab, Carcinus aestuarii [30], and the snow crab [9]. In the blue and green crabs, the morphology of white, blue, green, and red cuticles showed systematic differences at the ultrastructure level, and the red cuticle, which was found in the finger of the blue crab’s claw, showed the highest calcium concentration. In the snow crab, the hardness and elastic modulus of the regions (white) near the top of the claw were larger than those of the regions (red) near of bottom of the claw. In the mud crab, the microstructure, composition, and mechanical properties can change significantly where the color of the claw fingers changes, i.e., the discoloration line. We paid attention to the discoloration points and examined the correlation between the microstructure, composition, and local mechanical properties (hardness and elastic modulus) of the mud crab’s claw using optical microscopy (OM), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), and Vickers and nanoindentation tests. The results showed that the discoloration line was also found inside the claw finger exoskeleton and that the composition clearly changed near the discoloration line, which was significantly reflected in the mechanical properties.
Materials and methods
Specimen preparation
As samples, two large, adult, male mud crabs with rigid exoskeletons were obtained live from a local market in Naha, Okinawa, Japan. The mud crab shown in Fig. 1a was examined in detail. The body weight (BW), carapace width, and carapace length of this crab were 1,265 g, 167.3 mm, and 113.4 mm, respectively. The length, width, and thickness of the large right claw were 141.8 mm, 66.5 mm, and 39.5 mm, respectively. The claw length was greater than the carapace length, and the claw was very large. When the pinching force was measured with the device used in the previous coconut crab study [16], the value was 580 N. The present mud crab has huge/robust claws with a pinching force more than 46 times its BW.
The crab was frozen, transported to Tsukuba, and stored at − 18 °C prior to analysis. The crab was thawed under running water, and then, the fixed finger was cut with a saw. The sample was cut to fit within the dimensions of a 40-mm diameter mounting cup used in the embedding process. The sample in the embedding cup filled with epoxy (EpoFix Resin, Struers, Tokyo, Japan) was left to cure at room temperature for approximately 12 h. To ensure the epoxy’s penetration into the specimen voids, the sample was placed under vacuum for 600 s soon after the addition of epoxy. Then, the sample was ground with SiC papers, subsequently polished with 9, 3, and 1 μm diamond suspensions, and finally polished with a 0.05-μm alumina suspension. After polishing, cross-sectional micrographs of the sample were created by merging multiple OM images. Subsequently, the Vickers test and nanoindentation tests were conducted, and XRD was performed. After these were completed, the sample was coated with about 2 nm of osmium (Os) to characterize the microstructure and chemical composition [20, 26]; namely, the sample material consists of dry specimens cut from the right claw.
Vickers hardness testing
The Vickers test was conducted using a Shimadzu Micro Vickers Hardness Tester, HMV-2TADW (SHIMADZU, Kyoto, Japan), to understand the difference in hardness in some characteristic areas of the sample cross section. The tests were conducted five times for each area. The measurement was taken with an application of 98.07 mN for 15 s.
X-ray diffraction
The XRD analysis was carried out at ambient temperature to reveal the crystal structure in some characteristic areas. Microdiffraction experiments were performed using a commercial X-ray diffractometer, SmartLab (Rigaku Co. Ltd., Tokyo, Japan), equipped with a single photon-counting detector, HyPix-3000. The Cu Kα X-ray radiation (λ = 1.5418 Å) was generated by a Cu-rotator anode with an operating voltage and current of 45 kV and 200 mA, respectively. A micro-X-ray beam with a 100 μm diameter was shaped using a collimator system. Obtained two-dimensional diffraction images were then converted using 2DP software onto one-dimensional diffraction spectra with the step size of 0.02°. The measurement position for XRD was determined by observation camera and XY-movable stage with the accuracy of about 200 μm. The unit cell volume was calculated from the lattice parameter obtained from XRD data. For a hexagonal crystal system belonging to calcite, the unit cell volume is defined as V = SQRT(3)/2 * a^2*c, where a and c are the lattice parameters.
Microstructure and chemical compositions
For the microstructure characterization, a focused ion beam (FIB)–SEM dual-beam instrument (Scios2, Thermo Fisher Scientific, Waltham, MA, USA) was used. Before SEM observation, the sample surface was treated with an Os coating about 2 nm thick. An accelerating voltage of 2 kV was used as a typical SEM observation condition with a secondary electron detector in a chamber or an in-lens annular back-scattered electron detector. The energy-dispersive X-ray spectroscope attached to this FIB-SEM instrument was used for compositional analysis. A large silicon-drift detector (Oxford Instruments Ultim Max 170 EDS, Abingdon, Oxfordshire, GB) ensures high detecting efficiency and low statistical error in quantitative analysis. An accelerating voltage of 15 kV was used when the energy-dispersive X-ray spectroscopy (EDS) analysis was carried out.
Fracture surfaces
A fracture surface was observed to reveal the microstructures of the exoskeleton. The test piece for observing the fracture surface was cut from the movable finger with a saw, and then, the piece was placed in air for more than 48 h. The test piece was broken by hitting the back of a chisel with a hammer before SEM observation. Then, Os coating was applied to the fracture surface to obtain a clear microstructure image of organisms by eliminating the electron charge-up. The fracture surface was observed via SEM (JSM-7900F, JEOL, Tokyo, Japan; accelerating voltage: 2 kV; detector: Everhart–Thornley secondary electron detector (ET–SE)).
Nanoindentation tests
Nanoindentation testing was performed at ambient temperature using an ELIONIX, ENT-NEXUS (ELIONIX, Tokyo, Japan) with a Berkovich diamond indenter with an angle of 115°. The tests were conducted at intervals of 50 μm from an outer surface to an inner surface of sample cross sections. The loading curve consisted of a 5 s loading time, holding for 5 s at the maximum force of 5 mN, and then a 5 s unloading time. The hardness (H) and reduced elastic modulus (Er) were analyzed from the unloading curve using the Oliver–Pharr method employed in biological studies [8, 20, 22, 26].
Results
Microstructure, Vickers hardness, and crystal structures
The cross section of the fixed finger is shown in Fig. 1c. Interestingly, a boundary between the white region and the deep blue region seen on the outer surface of the exoskeleton was also found inside the claw exoskeleton. This discoloration point (DP) observed on the exoskeleton surface proceeded to the inside of the exoskeleton and was seen as a discoloration line (DL) near the mid-thickness of the exoskeleton. The OM image that clearly shows the DL is shown in Fig. S1 of supplementary information. Figure l d shows an enlarged image including the DP/DL, and Fig. l e shows an enlarged view near the exoskeleton surface with deep-mottled blue under the DP. The enlarged OM images show striation patterns originating from the twisted plywood structure patterns that have been observed in the exoskeletons of many crustaceans and the hammer of a stomatopod dactyl club [7, 22, 27, 31, 32]. These striation patterns were visible not only near the outer surface layer of the exoskeleton (Fig. 2b–e) but also near the inner surface layer (Fig. 2h–j) and the top (Fig. 2a). The striation patterns were clearly visible inside the deep blue surface exoskeleton under the DP as compared to inside the white surface exoskeleton above the DP. Curiously, tissue structures different from the wavy striation patterns were observed at the outer surface layer of the deep blue exoskeleton, as shown in Fig. 2e, f, g. These structures with a thickness of 45–100 µm existed irregularly along the surface layer, and the striation patterns changed significantly near these structures, as shown in Fig. 2f. Similar tissue structures have been observed in the dorsal carapace of the edible crab [22], where they denote the exocuticle layer, and the layer of striation patterns denotes the endocuticle. The inside of the exocuticle had a fish-scale-like tissue extending normally to the surface (Fig. 2g).
Figure 3a shows the Vickers hardness, HV0.01 = (average values) ± (standard deviations), at six characteristic areas in the claw cross section. Here, each area corresponds to a position 100–200 µm away from the outer/inner surfaces. In regions a, b, and c, the outer surface area was harder than the inner surface area. In particular, in regions a and b, which exist with the DL inside the exoskeleton, the hardness of the outer surface area is 2.5 times or more that of the inner surface area. On the other hand, in region c without the DL, the hardness of the outer surface area, c1, was 1.7 times that of the inner surface area, c2. The hardness of area c1 was considerably lower than that of areas al and bl, but that of area c2 was only slightly lower than that of areas a2 and b2. That is, although a similar striation pattern was observed from the outer surface to the inner surface, a large change in hardness existed, with the DL as the boundary. Such a difference in hardness was also seen in the results of the nanoindentation test, as shown in Fig. S2 of supplementary information.
a The hardness at six characteristic areas in the claw sample, obtained through Vickers tests. The tests were conducted five times for each area, and the areas are 100–200 μm away from the outer/inner surfaces. b XRD patterns of the six characteristic areas, including the reference XRD pattern taken from the calcite powder (FUJIFILM Wako Pure Chemical Corp.)
The results obtained by XRD are shown in Fig. 3b. Enlarged figures of some preferential diffraction angles are shown in Fig. S3 of supplementary information. The crystalline structures of these six characteristic areas were all calcite. The difference in peak intensity for each area is associated with the texture effect. Curiously, in regions a and b, with the DL, significant peak shifts to the high-angle side were observed from the outer surface (a1 and b1) to the inner surface (a2 and b2). Moreover, for instance, the diffraction peak widths of (104), (113), (202), and (116) of the inner surface are wider than that of the outer surface. On the other hand, no such peak shifts and significant differences in peak widths are visible in region c, without the DL. This suggests that the unit cell volume and crystallinity of the calcite structure change in the exoskeleton with the DL.
SEM micrographs from near the outer surface, center of thickness, and inner surface on Line B and Line C displayed in the claw cross section in Fig. 3a are shown in Figs. 4 and 5, respectively. On Line B with the DL shown in Fig. 4, the whole part of the exoskeleton with a 3000 μm thickness is parallelly striated with the stacking height (Sh) of the plywood layers, and the Sh gradually decreases as it approaches the outer and inner surfaces. In particular, the Sh near the inner surface is very small, 13 μm or less. The Sh near the outer surface and the DL was 15–25 μm. The contrast of the SEM images clearly changed, with the DL as the boundary; this is because the microstructure went from dense to coarse. Furthermore, the black spots (i.e., pore canals) which are not clearly seen on the outer surface layer increase from near the DL toward the inner surface layer. These many black spots were classified as streaks extending in the x-direction and small dots perpendicular to the x–y plane. On the other hand, on Line C without the DL, as shown in Fig. 5, a fish-scale-like tissue, as shown in Fig. 2g, is observed near the surface, and subsequent microstructures are striated with the Sh of the plywood layers, similarly to Line B. The Sh near the outer surface is very small, 4 µm or less; it increases as it approaches the thickness center and then decreases. The many black spots were visible from x = 96 μm to the inner surface layer, and clear tissue changes at the DL, as shown in Fig. 4, were not observed in the exoskeleton thickness on Line C. Figure 6 shows the SEM micrographs of areas perpendicular and parallel to the surface of the DL that proceeded from the DP to the inside of the exoskeleton. It can be seen that tissue changes on the DL parallel to the surface, as shown in Figs. 4 and 6c, d, are also observed on the DL perpendicular to the surface, as shown in Fig. 6 e, f.
SEM micrographs of Line B, shown in Fig. 3a. Here, DL denotes the discoloration line
SEM micrographs of line C, shown in Fig. 3a
Chemical compositions
The EDS results for the six areas shown in Fig. 3a are summarized in Fig. 7. Calcium (Ca), magnesium (Mg), phosphorus (P), carbon (C), and oxygen (O) were the main components, and aluminum (Al), sodium (Na), chloride (Cl-), nitrogen (N), and sulfur (S) were the minor amounts found in the analysis. Al is a residue from the alumina used in polishing. The other components may be due to the residual NaCl in mangroves and soil near the coast. These minor components are found in the exoskeleton of the coconut crab, edible crab, and lobster [21, 26]. N and S are presumed to be minor elements contained in organic compounds. The area scan results for Ca, C, Mg, and P for each area reveal the difference in compositions in the regions separated by the DL. Ca concentrations in areas a1 and b1 are higher than in other areas, but Mg concentrations are lower, and P concentrations are zero. These results show that the mineralization inside the claw is different from that in other areas. Figure 8 shows EDS results for inside the exoskeleton near the DP. The composition changes at the DL perpendicular to the surface can be observed in the circle charts and EDS maps. In particular, the EDS maps of inorganic matters, Ca, P, and Mg, shown in Fig. 8d clearly show the concentration change near the DL. These results are consistent with the results shown in Fig. 7.
Area scan results of EDS, including hardness HV0.01, shown in Fig. 3a, at six areas in the claw sample. Here, the EDS results show the average weight % of calcium (Ca), magnesium (Mg), phosphorus (P), carbon (C), oxygen (O), sodium (Na) and others
Fracture surface
Figure 9 shows SEM images of the fracture surface in the deep blue surface without the DL in the movable claw finger. The striation patterns observed in the endocuticle on the polished surface appeared as a terrace-step fracture surface, which consists of a terrace (//z) and a step (//x), on the fracture surface. Such a fracture surface effectively improves the fracture characteristics and provides mechanical resistance to avoid catastrophic failure [4, 5, 33,34,35,36]. The terrace-step fracture surface was often observed in the exocuticle with a twisted plywood–pattern structure on the fracture surface of the coconut crab exoskeleton [25, 26, 37]. The step height (//x) corresponds to the Sh of the plywood structure. The step height directly below the exocuticle shown in Fig. 9c is 4 µm, and it increases as it approaches the inside. The step height decreases as it approaches the inner surface, as shown in Fig. 9d, e. These tendencies are consistent with the SEM image shown in Fig. 5. Enlarged SEM images of the terrace-step fracture surface are shown in Fig. 10. The fracture surface of the endocuticle in the mud crab’s claw shows a twisted plywood–pattern structure, which have been observed in an exocuticle and endocuticle of many crustaceans and the scorpion’s pincer [2, 6, 7, 22, 23, 26, 27, 38].
Figure 11 shows SEM images of the fracture surface in the white surface exoskeleton with the DL. Although a terrace-step fracture surface was visible on the entire fracture surface, the exocuticle shown in Fig. 9a, b was not present on the surface layer. The Sh is 20–25 μm near the outer surface and 10–15 μm near the inner surface; these values are in agreement with the results of the polished surface observed in Fig. 4. However, since the white surface exoskeleton was strongly mineralized and very dense, the twisted plywood structure shown in Fig. 10 could not be clearly observed.
Discussion
One of the most notable features of the exoskeleton forming the huge claws of the mud crab is the fact that the DP seen on the outer surface of the exoskeleton of the claw top proceeded to the inside of the exoskeleton and was observed as a DL at the cross section of the 3.0–3.3-mm-thick exoskeleton (Figs. 1 and 2). The existence of this DL was confirmed in another mud crab (body weight: 1,578 g), as shown in Fig. S4 of supplementary information. The existence of such a DL inside the exoskeleton has rarely been reported in other crustaceans. Moreover, the exocuticle that existed irregularly along the surface in the deep blue surface exoskeleton was not in the white surface exoskeleton of the claw top (Figs. 2e–g, 9a, b, and 11. Although the tissue structure basically had a twisted plywood pattern throughout the exoskeleton thickness regardless of the DL, the chemical composition, density, crystallinity, and hardness varied significantly, with the DL as the boundary.
Exocuticle and endocuticle in the white surface exoskeleton
In many other crustaceans [20, 22,23,24,25,26,27, 31, 32], the exoskeleton is composed of the epicuticle, the exocuticle, the endocuticle, and the membranous layers; two layers of the exocuticle and the endocuticle are strongly mineralized and have a tissue structure with a twisted plywood pattern. However, in the exoskeleton of the coconut crab, the twisted plywood pattern was observed only in the exocuticle [25]. Usually, the exocuticle is strongly mineralized, harder and thinner than the endocuticle. The hardness changes abruptly at the boundary between these layers. In the coconut crab, the exocuticle thickness in the claw accounted for 10% of the exoskeleton thickness [25], and its proportion in other parts—the first walking legs, cephalothorax, and abdomen—was 14–19% [26]. The blue crab’s claw changes from red to white to blue from top to bottom; the exocuticle thickness was 2.4% for the red exoskeleton, 4.5% for the white exoskeleton, and 5.4% for the blue exoskeleton [30]. This proportion for the green crab’s claw with a green surface exoskeleton was 19% [30], and the proportion for the dorsal carapace of the edible crab was about 25% [22]. In the deep blue surface exoskeleton of the mud crab’s claw (Figs. 2, 5, and 9), the exocuticle was 45–100 µm thick, which accounted for 1.5–3.0% of the exoskeleton thickness of 3300 µm. The exoskeleton of the mud club’s claw is much thicker than that of other crustaceans, but the ratio of thickness for the exocuticle and exoskeleton is close to that of the blue crab [30]. On the other hand, in the white surface exoskeleton, there was no layer corresponding to the exocuticle, but a large change in mineralization existed with the DL as the boundary (Figs. 4, 6, 8). In short, the difference in the color of the exoskeleton’s surface is related to the mineralization inside the exoskeleton, and the exoskeleton has a different tissue structure depending on the color of the surface. Although the tissue structure in the white surface exoskeleton had a twisted plywood pattern throughout the thickness, the region from the outer surface to the DL (OS) was strongly mineralized and dense compared to that from the DL to the inner surface (IS). As a result, the OS was at least 2.5 times harder than the IS, as shown in Fig. 3a. If the difference in mineralization (e.g., Ca concentration and hardness) is defined as the difference between the exocuticle and endocuticle, in the white surface exoskeleton, the OS layer corresponds to the exocuticle, and the IS layer corresponds to the endocuticle. The exocuticle for region b was 1500–1700 µm thick, and its thickness accounted for 52–59% of the exoskeleton thickness of 2800–3000 µm. Moreover, in the claw tip corresponding to region a, this proportion was 72%, and most of the exoskeleton was occupied by the hard exocuticle layer. It is considered that the proportion of the hard exocuticle increased in the white surface exoskeleton in the claw top to maintain the function of claws that are huge in comparison with the body size of the mud crab.
Changes in mechanical properties
Based on the SEM images shown in Figs. 4 and 6, the microstructure appears to change gradually rather than abruptly at the DL. This change is different from that in the deep blue surface exoskeleton (Fig. 5) and the exoskeleton of the American lobster [31], the European edible crab [22], and the coconut crab [25]. Additionally, nanoindentation tests were performed along the exoskeleton thickness from the outer surface to the inner surface to investigate the variation of local mechanical properties that correspond to these microstructural changes. Figure 12 shows the distribution of H and Er along Lines B and C as displayed in Fig. 3a. Here, the tests were performed on two parallel lines 100 μm apart for regions b and c. In Line B, the H decreased with x from the outer surface, became constant (2.0 GPa) at x = 200 µm, subsequently started to decrease from x = 1500 mm and was in the range of 0.5–1.0 GPa in the endocuticle. The Er also showed the same change throughout the exoskeleton thickness; the mechanical properties gradually varied near the DL. This reflects the gradual microstructure change from dense to coarse near the DL, as shown in Fig. 4. Note that the data points near DL are relatively sparse. This may be related to the local differences in the grade of mineralization and microstructure of the intermediate layer (DL) between the exocuticle and endocuticle. On the other hand, in Line C, the H and Er in the endocuticle were slightly larger near the outer surface, but they were almost constant until x = 2500 µm thereafter and decreased near the inner surface adjacent to the cells. The values of the H and Er in the endocuticle on Line B match those in the endocuticle on Line C except near the inner surface. The H»2 GPa in the exocuticle on Line B is about 2.5 times than that of the H»0.8 GPa in the endocuticle; this result is consistent with the Vickers hardness shown in Fig. 3a. As displayed in Fig. 12 insets, the exoskeleton of the mud club’s claw, whose color changes are roughly divided into an exocuticle and endocuticle, with the DL as the boundary. The very thin exocuticle in the deep blue surface exoskeleton had a tissue structure that did not have a twisted plywood pattern, as shown in Figs. 2g and 5. We plan to investigate this in future.
Distributions of a hardness, H, and b elastic modulus, Er, with distance from the outer surface, x, on Lines B1, B2 and C1, C2 in the claw cross section. Here, Lines B1 and B2 and Lines C1 and C2 are parallel lines separated by 100 µm, respectively. The H(epo) and Er(epo) denote the hardness and elastic modulus of the cold epoxy resin, respectively
Changes in crystallinity and chemical components inside the exocuticle
As shown in Fig. 3b and S2, the crystal structures of the claw were calcite. However, there was a difference in crystallinity between the outer surface and the inner surface. To investigate the differences in crystallinity throughout the exoskeleton with and without the DL in more detail, XRD analysis along Lines B and C was performed using the same method as in Sect. 2.3. Figure 13 shows variations in the unit cell volume (V) with distance, x. Here, Lines B and C were measured from the outer surface to the inner surface at intervals of 200 µm. The Vs of areas a1 and a2 on the claw top were analyzed using the data shown in Fig. 3b. In Line B, the Vs decreased linearly to x = 1500 µm and changed abruptly at x = 1700 µm, corresponding to the DL. On the other hand, in Line C, there was no such abrupt change. The V in the highly dense areas (100–500 µm in Line B and area a1) just below the white surface exoskeleton is 364–365 Å3, and this value agrees with the unit cell volume of calcite reported by Althoff [39]. In Line B, the V decreases to 356 Å3 at the DL. Subsequently, the V increases to 358 Å3 with x and agrees with the V of Line C. That is, a clear change in V was shown only in the exoskeleton with the DL. In the field of minerals, research on the formation of dolomite, CaMg (CO3)2, has been carried out for a long time. In the equilibrium of calcium–magnesium carbonates [39], it is known that the cell unit volume decreases proportionally from calcite (V = 366 Å3) to magnesite (V = 280 Å3) as the Mg concentration increases. In other words, the ionic radius of Mg2+ (0.72 Å3) is smaller than that of Ca2+ (1.00 Å3) [40], so when a part of the Ca in the calcite structure is replaced with Mg, the V becomes smaller as the Mg increases. According to the composition analysis shown in Figs. 7 and 8, the main components of the exoskeleton changed with the DL. If Ca concentrations decreased in the exocuticle on Line B and, contrarily, Mg concentrations increased, the variation in V in Line B shown in Fig. 13 may be associated with the system CaCO3–MgCO3. To clarify this fact, additional EDS analysis was carried out for Line B from the outer surface to the inner surface. The result is shown in Fig. 14. As expected, the result reveals decreasing Ca concentrations from the outer surface to the DL in the exocuticle layer and a concomitant increase in Mg throughout the same layer. If hardness correlates only with the Ca concentration, the H shown in Fig. 12 should gradually decrease with x in the exocuticle on Line B. The change in V inside the exocuticle shown in Fig. 13 suggests that some of the Ca atoms were replaced with Mg atoms, and as a result, the crystallinity of calcite near the inner surface was weaker than that near the outer surface, as shown in Figs. 3b and S2. Moreover, this result means that P-related minerals such as calcium phosphate are not present in this region.
Variations in unit cell volume, V, of calcite structure obtained by XRD on Line B in the exoskeleton with the DL and Line C in the exoskeleton without the DL. Here, the V of calcite denotes the result reported by Althoff [36]
Conclusion
The exoskeleton of the mud crab’s huge claw was analyzed using a materials science approach. The boundary between the white region and the deep blue region seen on the exoskeleton surface was also found inside the exoskeleton, and the difference in claw coloration was related to the mineralization inside the exoskeleton. In the white surface exoskeleton, more than half of the exoskeleton thickness was occupied by a hard and dense exocuticle layer, which increased to 72% at the claw tip. The hardness of the exocuticle was 2.5 times that of the endocuticle, and the microstructures and mechanical properties varied gradually with the boundary between the exocuticle and endocuticle. The mechanical properties were almost constant in the exocuticle, but in that region, Ca concentrations decreased from the outer surface toward the boundary and Mg concentrations increased. The change in unit cell volume obtained by XRD suggested that some of the Ca atoms in the calcite structure were replaced with Mg atoms. The thick exocuticle and this graded change in the claw top should help to retain the function of the huge claws.
References
Islam MK, Hazell PJ, Escobedo JP, Wang H (2021) Biomimetic armour design strategies for additive manufacturing: a review. Mater Design 205:109730. https://doi.org/10.1016/j.matdes.2021.109730
Naleway SE, Taylor J, Porter MM, Meyers MA, McKittrick J (2016) Structure and mechanical properties of selected protective systems in marine organisms. Mater Sci Eng C 59:1143–1167. https://doi.org/10.1016/j.msec.2015.10.033
Huang W, Restrepo D, Jung J-Y, Su FY, Liu Z, Ritchie RO, McKittrick J, Zavattieri P, Kisailus D (2019) Multiscale toughening mechanisms in biological materials and bioinspired designs. Adv Mater 31:1901561. https://doi.org/10.1002/adma.201901561
Launey ME, Ritchie RO (2009) On the fracture toughness of advanced materials. Adv Mater 21:2103–2110. https://doi.org/10.1002/adma.200803322
Kakisawa H, Sumitomo T (2011) The toughening mechanism of nacre and structural materials inspired by nacre. Sci Technol Adv Mater 12:064710. https://doi.org/10.1088/1468-6996/12/6/064710
Currey JD, Nash A, Bonfield W (1982) Calcified cuticle in the stomatopod smashing limb. J Mater Sci 17:1939–1944. https://doi.org/10.1007/BF00540410
Huang W, Shishehbor M, Guarín-Zapata N, Kirchhofer ND, Li J, Cruz L, Wang T, Bhowmick S, Stauffer D, Manimunda P, Bozhilov KN, Caldwell R, Zavattieri P, Kisailus D (2020) A natural impact-resistant bicontinuous composite nanoparticle coating. Nature Mater 19:1236–1243. https://doi.org/10.1038/s41563-020-0768-7
Politi Y, Priewasser M, Pippel E, Zaslansky P, Hartmann J, Siegel S, Li C, Barth FG, Fratzl P (2012) A Spider’s fang: how to design an injection needle using chitin-based composite material. Adv Funct Mater 22:2519–2528. https://doi.org/10.1002/adfm.201200063
Zhang Y, Xu D, Li J, Zhang Z, Ding S, Wu W, Xia R (2021) Mechanical properties and clamping behaviors of snow crab claw. J Mech Behavior Biomedical Mater 124:104818. https://doi.org/10.1016/j.jmbbm.2021.104818
Luo Q, Nakade R, Dong X, Rong Q, Wang X (2011) Effect of mineral–collagen interfacial behavior on the microdamage progression in bone using a probabilistic cohesive finite element model. J Mech Behavior Biomedical Mater 4:943–952. https://doi.org/10.1016/j.jmbbm.2011.02.003
Wu K, Song Z, Zhang S, Ni Y, Cai S, Gong X, He L, Yu S (2020) Discontinuous fibrous Bouligand architecture enabling formidable fracture resistance with crack orientation insensitivity. PNAS 117:15465–15472. https://doi.org/10.1073/pnas.2000639117
Cheng L, Thomas A, Glancey JL, Karlsson AM (2011) Mechanical behavior of bio-inspired laminated composites. Compos A Appl Sci Manuf 42:211–220. https://doi.org/10.1016/j.compositesa.2010.11.009
Huber DR, Eason TG, Hueter RE, Motta PJ (2005) Analysis of the bite force and mechanical design of the feeding mechanism of the durophagous horn shark heterodontus francisci. J Exp Biol 208(18):3553–3571. https://doi.org/10.1242/jeb.01816
Alexander RM (1985) The maximum forces exerted by animals. J Exp Biol 115(1):231–238. https://doi.org/10.1242/jeb.115.1.231
Taylor GM (2000) Maximum force production: why are crabs so strong? Proc Biol Sci 267:1475–1480. https://doi.org/10.1098/rspb.2000.1167
Oka S, Tomita T, Miyamoto K (2016) A mighty claw: pinching force of the coconut crab, the largest terrestrial crustacean. PLoS ONE 11:e0166108. https://doi.org/10.1371/journal.pone.0166108
Paul AJ, Dawe EG, Elner R, Jamieson GS, Kruse GH, Otto RS, Sainte-Marie B, Shirley TC, Woodby D (eds) Crabs in cold water regions: biology, management, and economics. Report No. AK-SG-02–01. University of Alaska Sea Grant, Anchorage, AK, USA, 2002
Alberts-Hubatsch H, Lee SY, Meynecke J-O, Diele K, Nordhaus I, Wolff M (2015) Life-history, movement, and habitat use of scylla serrata (decapoda, portunidae): current knowledge and future challenges. Hydrobiologia 763(1):5–21. https://doi.org/10.1007/s10750-015-2393-z
Hepburn HR, Joffe I, Green N, Nelson KJ (1975) Mechanical properties of a crab shell. Comp Biochem Physiol 50A:551–554. https://doi.org/10.1016/0300-9629(75)90313-8
Inoue T, Oka S, Nakazato K, Hara T (2021) Structural changes and mechanical resistance of claws and denticles in coconut crabs of different sizes. Biology 10:1304. https://doi.org/10.3390/biology10121304
Boßelmann F, Romano P, Fabritius H, Raabe D, Epple M (2007) The composition of the exoskeleton of two crustacea: the American lobster Homarus americanus and the edible crab cancer pagurus. Thermochim Acta 463:65–68. https://doi.org/10.1016/j.tca.2007.07.018
Fabritius H-O, Karsten ES, Balasundaram K, Hild S, Huemer K, Raabe D (2012) Correlation of structure, composition and local mechanical properties in the dorsal carapace of the edible crab cancer pagurus. Z Kristallogr 227:766–776. https://doi.org/10.1524/zkri.2012.1532
Nikolov S, Petrov M, Lymperakis L, Friak M, Sachs C, Fabritius H-O, Raabe D, Neugebauer J (2010) Revealing the design principles of high-performance biological composites using Ab initio and multiscale simulations: the example of lobster cuticle. Adv Mater 22:519–526. https://doi.org/10.1002/adma.200902019
Wang Y, Li X, Li J, Qiu F (2018) Microstructure and mechanical properties of the dactylopodites of the chinese mitten crab (Eriocheir sinensis). Appl Sci 8:674. https://doi.org/10.3390/app8050674
Inoue T, Oka S, Hara T (2021) Three-dimensional microstructure of robust claw of coconut crab, one of the largest terrestrial crustaceans. Mater Des 206:109765. https://doi.org/10.1016/j.matdes.2021.109765
Inoue T, Hara T, Nakazato K, Oka S (2021) Superior mechanical resistance in the exoskeleton of the coconut crab, Birgus latro. Mater Today Bio 12:100132. https://doi.org/10.1016/j.mtbio.2021.100132
Qian Z, Yang M, Zhou L, Liu J, Akhar R, Liu C, Liu Y, Ren L, Ren L (2018) Structure, mechanical properties and surface morphology of the snapping shrimp claw. J Mater Sci 53:10666–10678. https://doi.org/10.1007/s10853-018-2364-7
Hamdi M, Nasri R, Li S, Nasri M (2019) Bioactive composite films with chitosan and carotenoproteins extract from blue crab shells: biological potential and structural, thermal, and mechanical characterization. Food Hydrocolloids 89:802–812. https://doi.org/10.1016/j.foodhyd.2018.11.062
Waugh DA, Feldmann RM, Schroeder AM, Mutel MH (2006) Differential cuticle architecture and its preservation in fossil and extant callinectes and scylla claws. J Crustacean Biol 26:271–282. https://doi.org/10.1651/S-2692.1
Nekvapil F, Pinzaru SC, Barbu–Tudoran L, Suciu M, Glamuzina B, Tamas T, Chis V (2020) Color-specific porosity in double pigmented natural 3D-nanoarchitectures of blue crab shell. Sci Rep 10:3019. https://doi.org/10.1038/s41598-020-60031-4
Raabe D, Romano P, Sachs C, Al-Sawalmih A, Brokmeier H-G, Yi S-B, Servos G, Hartwig HG (2005) Discovery of a honeycombstructure in the twisted plywood patterns of fibrous biological nanocomposite tissue. J Crystal Growth 283:1–7. https://doi.org/10.1016/j.jcrysgro.2005.05.077
Yano I (1972) A histochemical study on the exocuticle with respect to its calcification and associated epidermal cells in a shore crab. Bull Jpn Soc Sci Fish 38–7:733–739. https://doi.org/10.2331/suisan.38.733
Inoue T, Yin F, Kimura Y, Tsuzaki K, Ochiai S (2010) Delamination effect on impact properties of ultrafine-grained low-carbon steel processed by warm caliber rolling. Metall Mater Trans A 41:341–355. https://doi.org/10.1007/s11661-009-0093-x
Pozuelo M, Carreno F, Ruano OA (2006) Delamination effect on the impact toughness of an ultrahigh carbon–mild steel laminate composite. Compos Sci Technol 66:2671–2676. https://doi.org/10.1016/j.compscitech.2006.03.018
Deville S, Saiz E, Nalla RK, Tomsia AP (2006) Freezing as a path to build complex composites. Science 311:515–518. https://doi.org/10.1126/science.1120937
Inoue T, Ueji R (2020) Improvement of strength, toughness and ductility in ultrafine-grained low-carbon steel processed by warm bi-axial rolling. Mater Sci Eng A 786:139415. https://doi.org/10.1016/j.msea.2020.139415
Inoue T, Oka S, Nakazato K, Hara T (2022) Columnar structure of claw denticles in the coconut crab, Birgus latro. Minerals 12:274. https://doi.org/10.3390/min12020274
Kellersztein I, Cohen SR, Bar-On B, Wagner HD (2019) The exoskeleton of scorpions’ pincers: structure and micro-mechanical properties. Acta Biomater 94:565–573. https://doi.org/10.1016/j.actbio.2019.06.036
Althoff PL (1977) Structural refinements of dolomite and a magnesian calcite and implications for dolomite formation in the marine environment. Am Miner 62(7–8):772–783
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767. https://doi.org/10.1107/S0567739476001551
Acknowledgments
We would like to thank Dr. Hara for his advice on observing microstructures and Ms. Kashihara for creating the illustrations.
Funding
This research was funded by JSPS KAKENHI Grant Number JP21H04537. The grant is greatly appreciated.
Author information
Authors and Affiliations
Contributions
The following statements should be used. TI was involved in conceptualization, methodology, investigation, visualization, project administration, supervision, funding acquisition and writing—original draft preparation; TI and TH contributed to formal analysis;; KN and SO were involved in resources; TI, TH and YH contributed to data curation;; TI, TH and SO were involved in writing—review and editing,; ;. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inoue, T., Hiroto, T., Hara, Y. et al. Tissue structure and mechanical properties of the exoskeleton of the huge claws of the mud crab, Scylla serrata. J Mater Sci 58, 1099–1115 (2023). https://doi.org/10.1007/s10853-022-08083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-08083-x