Abstract
In this work, copper and zirconium were added into the Ti(Al)N matrix to form deposited on TiAl(Cu, Zr)N coating using RF magnetron sputtering system in order to improve the mechanical, wear performance, and antibacterial activity. Results revealed that the TiAl(Cu, Zr)N coatings have a dense structure with (111) preferential orientation. The deposited TiAl(Cu, Zr)N coatings exhibited fcc-TiN structure, whereas the addition of Zr and Cu into the Ti(Al)N induced the appearance of Cu and ZrN. The solid solution strengthening and the increase in compressive residual stress (− 2.54 GPa) of Ti(Al, Zr)N coating with higher lattice strain (+ 0.1520) by the addition of Zr improved the hardness from 20.9 GPa. For the tribological property, the friction coefficient and wear rate decreased from (0.45, 5.9 × 10−3mm3/Nm) for the TiAlN to (0.29, 4.2 × 10−5mm3/Nm) for the Ti(Al, Zr)N, respectively, with high elasto-plastic H/E, H3/E2 (0.076, 0.121 GPa) ratios due to the low surface roughness, high adhesion, and nanocomposite structure. The results indicated the addition of Zr improves the friction property of Ti(Al)N coating. Although with their lower hardness than Ti(Al)N coating, the Ti(Al, Cu)N coating with 9.2 at.% Cu exhibited an excellent wear resistance and good antibacterial activity, which demonstrates its potential as a candidate material for biomedical application.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of metallic biomaterials to replace failed hard tissues has increased substantially due to the ageing world population. Stainless steel (SS) with a low carbon and a high chromium content is known for its manufacturability, cost-effectiveness, excellent mechanical properties, high resistance to high-temperature creep, good formability, resistance to corrosion and oxidation, and biocompatibility without allergic problems. These excellent characteristics have attracted the attention of researchers towards the use of this material in the biomedical field, such as artificial joints, stents, and dental implants and make this material the most used biomaterial [1,2,3].
However, further improvements are required to tackle corrosion problems propagating in the tissue surrounding the implant, the limited mechanical strength and the reduced tribological performance [4]. Titanium and titanium-based alloy coatings, in addition to heat treatment [1], are an often-used solution to overcome the aforementioned obstacles. Also, binary and ternary titanium nitrides have been applied to enhance the surface properties of 316L stainless steel [5,6,7]. The smaller covalent radius of aluminium (0.143 nm) compared to the one of titanium (0.146 nm) facilitates its insertion into the TiN lattice to form a ternary TiAlN nitride with the same lattice type. This material is used as a metastable hard coating for cutting and forming tools, in semiconductor devices, decorative and biomedical applications [8,9,10,11]. The TiAlN system exhibits outstanding features such as excellent mechanical and tribological proprieties, especially low coefficient of friction, high hardness, and good toughness that significantly increase its erosion resistance [12,13,14,15,16]. In addition to its biocompatibility and compared to other coatings, TiAlN has shown remarkable improvements in fatigue strength, good optical quality, good wear, and chemical stability, low thermal conductivity and better oxidation resistance [17,18,19,20,21]. Moreover, TiAlN coatings are typically dense with strong adhesive properties. Grown on a metal surface, it protects the substrate from oxidation. The formation of wurtzite AlN leads to a significant reduction in the mechanical properties of TiAlN coatings [20], but this process occurs only at higher temperature than typically used for biomedical applications.
The TiAlN system has been doped with different elements such as Si [22], Ta [23], O [24], C [25], V [26], Ag [27], and Cr [28] to induce grain refinement and limit the dislocation activity. For example, by an optimization of the carbon content, Zhang et al. [25] improved the hardness and lowered the friction coefficient of TiAlCN (2443 HV25g). Tillmann et al. [30] used direct current magnetron sputtering (DCMS) and high-power impulse magnetron sputtering (HiPIMS) techniques to elaborate TiAlN and TiAlCN coatings on quenched and tempered AISI H11 tool steels. They found that the insertion of carbon into TiAlN led to reduce the compressive residual stresses from (− 1047 ± 149) to (− 307 ± 211) MPa in DCMS-deposited films and from (− 7035 ± 1361) to (+ 989 ± 187) MPa in the HiPIMS-deposited films. Carbon also reduced the friction coefficient of coating. Wang et al. [26] examined the effect of vanadium addition on the microstructure of TiAlN coating deposited on AISI M2 high-speed steel substrates by magnetron reactive sputtering. The TiAlVN film showed a columnar structure with a (111) preferred orientation. The addition of V increased the lattice constant of TiAlN crystal and improved its hardness.
Some studies evaluated the effects of Zr and Cu on the TiAlN coating properties [27,28,29,30]. For applications as coatings for surgical and dental instruments, Mejía et al. [27] evaluated Ag- and Cu-doped TiAlN coatings (11 to 20 at.%) deposited on AISI 420 steel by unbalanced DC magnetron sputtering. This latter study demonstrated a higher antibacterial effect of doped coatings against S. aureus and E. coli bacteria. Chen et al. [28] studied the effect of Zr on microstructure, mechanical and thermal properties of magnetron sputtered TiAlN coatings. The addition of Zr up to 17 at.% increased the lattice parameter from 4.18 to 4.29 Å and improved the hardness from approximately 33 to 37 GPa. Moreover, an even higher hardness value of approximately 40 GPa was achieved by thermal annealing.
A combined investigation on the both antibacterial activity and tribo-mechanical properties of the Ti(Al, Cu)N and Ti(Al, Zr)N systems, which have the potential to protect biomaterials, is to the best of our knowledge lacking in the literature. Furthermore, the structure, mechanical, tribological, and corrosion performance of Ti(Al)N films as a function of (Zr, Cu) contents has been presented without considering the effect of surface morphology and preferential orientation. Nevertheless, hence, in this work the effects of incorporating Cu and Zr into Ti(Al)N coatings deposited on AISI 316L stainless steel by magnetron sputtering were investigated.
Hence, in this work, the evolution of chemical composition, orientation, crystallite size, microstrain, and surface roughness is provided for these TiAl(Cu, Zr)N films as well as the correlation with their mechanical, tribological properties and antibacterial activity was investigated. As well as the comparative study between the role of Cu and Zr in strengthening the intergranular bonding of Ti(Al)N systems to enhance its behaviour was investigated.
Experimental methods
Ti(Al, Cu)N and Ti(Al, Zr)N coatings were deposited on mirror-polished 316L stainless steel (AISI 316L SS) disc substrates (∅25 × 8 mm) and Si (100) wafers (10 × 10 × 480 μm) using RF magnetron sputtering (DEPHIS 4 PVD machine, Etupes, France) using pure Ti (Ø 10 × 1 mm), Al (Ø 10 × 1 mm), Cu (Ø 10 × 1 mm) and Zr (Ø 10 × 1 mm) targets. The elemental composition (wt. %) of the used 316L stainless steel (316L SS) samples was Cr (20–21), Ni (6–7), Mo (1.5), Mn (1.5), Si (1), P (0.04), C (0.03), S (0.03) and Fe (balance) [31]. The AISI 316L SS substrates were polished using SiC emery papers (400–1200 grit), followed by wet polishing in a diamond slurry. The average roughness (Ra) of the mirror-polished substrates was 0.03 μm. After polishing, the substrates were cleaned in acetone and subsequently in ethanol ultrasonic bath for 10 min. Substrates were then washed with distilled water and dried by air. Before deposition, the substrates were placed on the substrate holder at a distance of 10 cm from the targets and cleaned by sputtering with Ar gas for 10 min at 200 W to remove impurities and surface oxides. The four targets were also sputter cleaned in Ar (100 sccm and 0.4 Pa) for 10 min at constant power: Ti (220 W, 1 A), Al (242 W, 1 A), Cu (315 W, 1 A), Zr (233 W, 1 A). After this cleaning procedure, the Ti(Al)N, Ti(Al, Cu)N, and Ti(Al, Zr)N coatings were deposited at a total working pressure of 0.4 Pain Ar/N2 mixtures. The argon (80 standard cubic centimeters per minute (sccm)) and nitrogen (20 sccm) flow rates were controlled by flow controllers (HORIBASTEC (Ether CAT, N100). Experiments were performed at constant power: Ti (670 W, 3 A), Al (140 W, 0.5 A), Cu (187 W, 0.5 A), Zr (125 W, 0.5 A). The rotation of substrate-holder was set at 10 rpm, and deposition was carried out at floating temperature for 3 h without heating the substrates. The approximate deposition rates were 0.68 μ/h for Ti(Al)N, 0.71 μ/h for Ti(Al, Cu)N and 0.78 μ/h for Ti(Al, Zr)N coatings, respectively. During the deposition films, the temperature of the chamber slightly increased with increasing the deposition time to achieve 68 °C in the last stage of the film pulverization due to the high ion bombardment of different elements. Thus, the obtained temperature was registered just after the deposition process finished.
The surface morphology and film microstructure were examined using a field emission scanning electron microscope (FE-SEM, Hitachi S3500 N, USA). Chemical compositions of thin films were analysed with energy (EDS) and wavelength dispersive X-ray spectroscopy (WDS) (Oxford INCA x-act, 15 kV, USA). The film texture was determined by X-ray diffraction (Bruker, Karlsruhe, Germany, 40 kV/40 mA, CuKα radiation) over a 2θ range from 30 to 80°. All the reflections were compared to standards gathered by the Joint Committee on Powder Diffraction and Standards (JCPDS, card #01–074-8388 for TiN, #01–070-0354 for AlN, #00–004-0836 for Cu, #00–47-1088 for Cu3N and #00–065-7723 for ZrN). The average crystallite size was determined by using the Scherrer equation [32].
The lattice parameter was calculated by the Bragg formula, as obtained from their peak positions.
By using the Bragg equation:
where θ is the angle between the normal of the diffracting plane and the incident X-ray, λ is the wavelength of the X-ray. In our case Cu Kα = 1.5405 Å, and n is the order of the diffracted beam.
The lattice parameters of TiN films were calculated using Miller indices (hkl) according the formula for the cubic structure]:
The lattice strain was determined using the following equation:
where \(\varepsilon\) is the lattice strain in the direction of the a axis, \(\Delta a=\left({a}_{film}-{a}_{bulk}\right)\), afilm and abulk of the cubic TiN phases.
The surface roughness was measured by an Atomic Force Microscope (AFM 100, APE research) with a scanning range of 3 × 3 μm2 under contact mode. The film residual stresses were determined from the substrate curvature radii of iron sample (50 × 3 × 0.2 mm3), which was attached on one side with a Neycotape (KAPT6338-12B) on the substrate-holder before and after film deposition by using Stoney’s formula [33].
The values of hardness and elastic modulus of the coatings were obtained by nanoindentation (TI 980 triboindenter, Bruker, Minnesota USA). The indents were performed using a Berkovich diamond tip at a maximum applied load of 8000 mN at which the maximum indentation depth was in the 150–180 nm range. The penetration depth of the indenter was limited to 7% of the total film thickness in order to avoid the influence of the substrate. In order to interpret the data accurately, 3 measurements were performed for each coating, and their averages were calculated. The area function of the nanoindenter was calibrated on a quartz standard sample based on the Oliver–Pharr’s model [34].
Adhesion strength of coatings was evaluated by a scratch Millennium 200 tester. Five measurements were carried out using a conical diamond tip with a 0.2 mm radius. The applied load increased from 0.01 to 60 N at a constant loading speed of 0.01 mN/s. The stripping speed and length were 10 mm/min and 5 mm, respectively. The morphology of worn samples was observed by means of an optical microscope.
The tribological properties of the coatings were investigated by sliding wear tests using a tribometer under a ball-on-disc configuration (CSM Instruments, Anton Paar, Peseux, Switzerland). 100Cr6 steel counterpart balls of 6 mm diameter were used with various normal loads (1, 3 and 5 N). All tests were conducted under the following conditions: dry sliding, room temperature, relative humidity of approximately 25 ± 3% and sliding speed of 50 rpm. The wear rates of all coatings were determined from the worn tracks using the following equation:
where Wr is the wear rate, V the wear volume (mm3), P is the applied load (lN), and L is the total sliding distance (m). The worn surfaces profiles were obtained using a 3D profilometer (AltiSurf 500, ALTIMET, Marin, France). The worn surfaces were analysed using aforementioned scanning electron microscope.
The antibacterial activity of coatings was assessed by using the following bacteria: gram negative: Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis), and gram negative: Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa). The bacterial inoculums were prepared from cultures aged 18 h; colonies were picked to obtain physiological water suspensions adjusted to 1 × 108 colony-forming unit/mL (optical density = 0.08, λ = 625 nm) [35]. In order to make the bacteria agents’ penetration faster, Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N thin films deposited on glass substrates were scraped by using a sharp medical scalpel. The tested solution was prepared by dissolving 4 g of the each scraped coating on dimethyl sulfoxide 1 ml (DMSO 40%) with 0.5 ml acetic acid (CH3COOH, 4%) in tubes and shaken for 30 min to get a clear suspension. To avoid any effect on the bacterial growth, the DMSO concentration was verified after testing the concentration range of 5 to 50%. An adapted version of the Bauer’s method [36] was used. Instead of paper discs used by Bauer, 6 mm-diameter agar cylinders cut from the Muller Hilton medium were used. The agar cylinders were impregnated with 15 μl of Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N suspensions. The control disc was impregnated with DMSO (40%), and the acetic acid solvent was then deposited on the surface of Muller Hilton culture medium previously inoculated. The calibrated inoculums of the bacteria culture were tested. After incubation for 18 h at 4 °C to allow the suspension to diffuse into the agar, the Petri dishes were incubated at 37 °C for 24 h and then examined, and the diameters of the inhibition zones surrounding the cylinders were measured.
Results and discussion
Microstructural and chemical characterization
The thickness of the deposited coatings was in the 2.05–2.35 μm range. Figure 1 shows the microstructure and the surface morphology of the deposited thin films. All films present columnar growth. The Ti(Al)N coating surface shows a large pyramidal structure. The addition of Cu and Zr results in finer columns. Qualitatively, it seems that especially the addition of Zr results in a much smoother surface with finer columns.
The effect of the Cu and Zr addition on the surface roughness was quantitatively evaluated using an AFM (Fig. 2).
The Ti(Al, Cu)N film presents a dense structure with globular sharp, while the Ti(Al, Zr)N coating presents a lamellar form. The Ti(Al)N and Ti(Al, Cu)N coatings had an average roughness of Ra = 7.21 ± 0.5 and 8.93 ± 0.5 nm, respectively. It is most probable that the increase in surface roughness and the grain refinement with the increase in the film thickness of Ti(Al, Zr)N coating is due to the shadowing effect, which originates from deposition of bigger Zr atoms on surface. However, the smooth surface was observed for the Ti(Al, Cu)N coating (Ra = 2.12 ± 0.5 nm). The addition of Cu, the film slightly increases and the surface becomes fine and dense which can repair the substrate surface and has a relatively smooth surface.
Table 1 summarizes the results obtained by XRD and the (EDS, WDS) analysis of TiAl(Cu, Zr)N nanocomposite coatings. The EDS/WDS analysis showed that under the given conditions approximately 10 at.% of Cu and Zr were added to the coatings. All deposited coatings were stoichiometric which can be confirmed from the calculated stoichiometry ratio Me/N defined as the ratio between the concentrations of metals (Me) and nitrogen. For all coatings, Me:N was close to one.
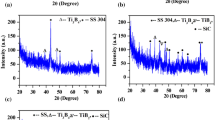
The TiAlN and TiAl(Cu, Zr)N nanocomposite coatings were studied with X-ray diffraction, and the obtained diffractograms are presented in Fig. 3. All coatings have an fcc-cubic structure with (200), (111), (220), (222) and (311) Bragg reflections which should belong to TiN. The TiAlN coating presents a (200) preferential orientation with a slight lattice strain (ɛ = 0.0042) and a low lattice parameter (a = 4.2529 Å) as compared to the bulk TiN value (abulk = 4.235 Å). This confirms that the mixture between Ti and Al produced a relaxed TiAlN film.
Compared to the Ti(Al)N coating (Fig. 3a), the XRD spectrum of Ti(Al, Cu)N coating (Fig. 3b) shows two additional peaks at 43.65° and 51.01° which can be attributed to the (111) and (200) Bragg reflection of Cu.
The Ti(Al, Zr)N coating spectrum (Fig. 3c) reveals small diffraction peaks at 33.3°, 39.5° and 56° assigned to ZrN (111), (200) and (220) (JCPDS card #00–065-7723). Despite the low Zr content (12.2 at. %), the formation of Zr in the Ti(Al, Zr)N is due to the affinity of Zr to the nitrogen that confirmed in our previous study [2].
Based on the XRD diffractograms, the texture coefficient, Tc = I111/(I111 + I200), was calculated. Compared to TiAlN (TcTiAlN≈0.46), texture coefficient was little affected by the Cu addition (TcTi(Al, Cu)N≈0.49) while it strongly increased with the addition of Zr (TcTi(Al, Zr)N≈ 0.74).
The lattice parameter a increased with the addition of both Cu (aTi(Al, Cu)N = 4.6271 Å) and Zr (aTi(Al, Zr)N = 4.8790 Å) as compared to the Ti(Al)N coating (aTiAlN = 4.2529 Å) by 1.09 and 1.15%, respectively. This change from the (200) plane towards the (111) preferential orientation was attributed to the re-nucleation of Ti(Al)N according to the (111) orientation with small amount of Zr as proposed by Abadias et al. [37]. The change in the preferential orientation was also due to the change in the film growth and grain orientation where a more compact structure could be found [38, 39].
According to Hongbo et al. [40], the lattice distortion induced by the solution of additional atoms can influence the residual internal stress of the films. Therefore, the diffraction peak shifting to a higher angle, which is attributed by the solution of the TiAlN with Zr or Cu, leads to the compressive internal stress. The (111) peak shift to lower 2θ values was observed for the Ti(Al, Zr)N coating. The Zr addition led to increase the lattice distortion and strain in coating from (ɛ = 0.042 in Ti(Al)N) to (ɛ = 0.1524 in Ti(Al, Zr)N). This is attributed to the increase in the lattice parameter related to the incorporation of Zr atoms (atomic radius = 1.60 Å) which are bigger than Ti and Al ones [39,40,41,42].
The crystallite size as calculated from the FWHM of (111) peak using the Scherrer equation was approximately 65.5, 21.4 and 15.4 nm for the Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N coatings, respectively. Obviously, the crystallite size was decreased with the addition of Cu and Zr. Hence, it seems that these elements act as grain-refiners. Thus, the decrease in the crystallite size of Ti(Al, Cu)N and Ti(Al, Zr)N coatings can be explained by the hindering of TiAlN crystal growth with the incorporation of Cu and Zr and the increase in nucleation sites.
Furthermore, in the Ti(Al, Cu)N and Ti(Al, Zr)N coatings, the stacking of atoms in the compact (111) preferred crystallographic plane enhances the sticking probability and blocks grain growth [43, 44]. The coating behaviour can be attributed to the high strain generated in the crystal and the changes in crystallite size and surface roughness related the addition of Cu and Zr.
Mechanical properties
The residual stress arising in the films during deposition was measured by the curvature method. The TiAl(Cu, Zr)N coatings were in a compressive stress state in the range of −1.65 to −2.54 GPa (Table 2). An increase in the (111) peak intensity is observed concomitantly with an increase in its width, probably due to the decrease in the crystallite size and the increase in residual stress in the Ti(Al, Zr)N. The compressive stresses developed in the Ti(Al, Zr)N coating are associated with the densification of the film by the addition of Zr. Therefore, the lattice distortion of the Ti(Al, Cu)N and Ti(Al, Zr)N. The compressive stress occurs by the incident of large Zr atoms from the film surface of with high energy. These atoms can be embedded in the grains forced into small spaces, causing lattice distortions which confirmed by increase in the lattice constant and with positive lattice strain (ɛ = + 0.1480) and inducing compressive stress–strain fields in the surrounding matrix.
Figure 4 shows typical load-penetration depth curves for the three Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N coatings. The continuous loading and unloading curves are relatively close to each other, indicating that coatings are elastic and there is no fracture or delamination at the surface under the applied loads [45,46,47]. In comparison with Ti(Al)N (149 nm at 6000 mN), the indenter penetration at the same load is similar for the Ti(Al, Cu)N film, but the Ti(Al, Zr)N coating was more resistant to indenter penetration (lower depth at higher load). The coating hardness (H) and elasticity modulus (E) values obtained from the nanoindentation measurements are summarized in Table 2. According to previous study, the TiAlN films have a high hardness [17,18,19,20,21], and the low hardness in Ti(Al)N film (15 GPa) can be due to preferential the lower thickness and a rough surface, resulting in a lower density of the Ti(Al)N coating which contributes to the dislocation motion across the large grain size. The addition of Cu slightly lowered the hardness and elasticity modulus values as compared to Ti(Al)N coating. Higher values were obtained for the Ti(Al, Zr)N coatings. Based on the hardness and the elasticity modulus, the elasto-plastic strain to failure of the coatings represented through the H/E and H3/E2 ratios is also presented in Table 2. It can be noticed that the Ti(Al)N coating has a low hardness (15.1 ± 1.6 GPa), which is related to the low packed (200) preferred orientation and the large grain size causing the softness of film, as described earlier [47, 48]. The Cu addition slightly decreased the hardness and elasticity modulus values from (15.1 ± 1.6 GPa, 189 ± 11 GPa) to (13.8 ± 1.6 GPa, 153 ± 11 GPa), respectively. The mechanical regression occurred due to the low texture with a slight decrease in I111/(I111 + I200) for the Ti(Al, Cu)N coating and the precipitation of ductile Cu metal [28, 49] confirms the XRD results presented in Fig. 3b. Hardness reduction in the Ti(Al, Cu)N film with the addition of Cu above 9.2% is mainly attributed by the amount of soft Cu phase as observed by Chenkai et al. [49] for the Mo2/Cu films. In fact, copper is immiscible with Ti(Al)N and can form separate grains that can easily deform under external loading favouring the intergranular deformation by sliding of grain boundary [46]. Furthermore, the mixed character of metallic (Me‐Me) with covalent (Me‐N) components is expected to have reduced the hardness.
However, the measured hardness of the Ti(Al, Cu)N coating was higher than that of copper-based nitride films (5–12 GPa). This is explained by the presence of hard Ti(Al)N phases and grain refinement in accordance with the Hall–Petch relationship [46, 50].
The maximum hardness and elasticity modulus of 20.9 ± 1.5 and 275 ± 12 GPa were obtained for Ti(Al, Zr)N coating. The presence of ZrN in the Ti(Al)N produced a dense nanocrystalline structure with fine grains that led to enhance the Ti(Al, Zr)N coating hardness according to Hall–Petch relationship [50]. The enhancement of the mechanical properties of coating can be attributed to the strengthening of crystallites by ZrN solid solution incorporated into Ti(Al)N, which reduces the crystallite size and promotes the grain boundaries inhibiting the dislocation movement [28]. This is accompanied by intragranular damage caused by lattice defects and dislocation aggregates, leading to an increase in compressive strain [51], favourably enhancing the hardness of the coating. In addition, Yang et al. [52] found that Ti(Al, Zr)N nanocomposite coatings had a higher hardness with the addition of Zr. They reported that the addition of Zr into the Ti(Al)N coatings limited the solubility of Ti(Al)N with Zr and consequently improved the mechanical properties. On the other hand, Yang et al. [52] pointed out the dislocation movement hindered by the nanosized grains in Ti(Al)N coating with the addition of Zr. Therefore, the sliding across grain boundaries becomes more difficult as the degree of coherency increases. The higher hardness of the Ti(Al, Zr)N coating was interpreted by the change in the diffraction peaks intensity from (200) to the hardest (111), as detected by XRD, due to the fact that Schmid factor is zero in all slip systems [52,53,54]. Furthermore, a rise in the elastic modulus of the Ti(Al, Zr)N coating was determined by the high slope of the unloading curves (Fig. 4b). The elasto-plastic strain to failure of the TiAl(Cu, Zr)N coatings, represented through the H/E and H3/E2 ratios, shows that the Ti(Al, Zr)N coating has the maximum H/E and H3/E2 ratios (0.076 ± 0.009 and 0.121 ± 0.041, respectively) (Fig. 4b). This reveals the beneficial role of the Zr in inhibiting the cracks propagation, and thus improving the elasto-plastic deformation of the films.
Tribological properties
Adhesion
Figure 5 illustrates the applied loads and failure modes during scratch tests carried out on Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N coatings. The scratch direction was from left to right for all films, and the critical load was determined from the change in the depth profile and acoustic emission corresponding to film delamination. Three critical loads including Lc1 (adhesive failure), Lc2 (adhesive and cohesive failure), and Lc3 (total coating failure) were defined [53]. The Ti(Al)N coating showed a significant amount of debris accumulated on the wear track, and adhesive failure was obtained at a low critical load Lc1 = 6.94 N. A complete delamination of the coating was detected in the first stage of the scratch path, with some pits observed within the film track (Fig. 5a). The film abrasion is related to its low resistance to plastic deformation, which is expected for ductile films [55]. The Ti(Al, Cu)N coating failed when some cracks were initiated inside the track (Lc1 = 6.46 N) due to low resistance to plastic deformation (Fig. 5b) [56, 57]. Belo et al. [58] attributed the low wear resistance of the TiCuN coating to the incorporation of Cu into the coating. They explained that by the reduction in the hard-TiAlN phase fraction and the Cu precipitation. A gradual increase in load resulted in tensile cracking and film delamination on the inner side of the track during scratching. The load values were determined as Lc2 = 35.25 N and Lc3 = 39.96 N.
It is clear that the Ti(Al, Zr)N coating with rougher surface shows greater cohesive/adhesive critical loads than the smooth Ti(Al, Cu)N coating. In fact, its high roughness has made it possible to create mechanical anchoring sites, thus strengthening its adhesion to the substrate. The Zr addition had a positive effect and contributed to high adhesion of the thin film (Fig. 5c). Furthermore, an addition of Zr led to the formation of a nanostructure that resisted cracks propagation at grain boundaries, as described earlier [59,60,61]. The high hardness and H3/E2 values resulted to reduce the plastic deformation at the asperities during scratch test and therefore reduced the contribution of work force to the delamination of the film. Yang et al. [52] observed a similar phenomenon when incorporating Zr into Ti(Al)N coatings and attributed this to high hardness and the reduced metastable solubility limit. With further increase in the applied load (Lc3 = 42.93 N), tensile cracks propagated through the coating and then caused delamination. These results might suggest that the adhesion strength is not only due to the good mechanical properties but also related to the texture and morphology of the deposited film.
Wear and friction
Figure 6 illustrates the evolution of coefficient of friction (CoF) of Ti(Al)N, Ti(Al, Cu)N and Ti(Al, Zr)N nanocomposite coatings tested under different normal loads of 1, 3 and 5 N. The coating CoF showed an initial run-in process and reached a steady state after a short sliding distance (about 80 m). This can be explained in relation to the contact area between the friction surfaces. At the initial stage, the contact area is small, pressure is high, and sliding is difficult and leads to an instable CoF due to the uneven geometry of friction surfaces. As the sliding distance increases, the contact area increases, the pressure decreases, and the CoF reaches a stable state [62,63,64,65]. On the other hand, for all coatings we noticed an increase in COF with increasing the normal load.
From the CoF curves obtained at 1, 3 and 5 N loading, large variations were observed during the sliding of the 100Cr6 balls on the film surface. At a normal load of 1 N, it can be seen that Ti(Al)N coating had the highest CoF (0.45). It was observed that the COFs of Ti(Al, Cu)N and Ti(Al, Zr)N coatings were lower than that of the Ti(Al)N coating, which can be the reason of high wear resistance of the coatings. A comparable tendency was also observed in the wear rates of coatings presented in Fig. 7. Accordingly, the Ti(Al, Cu)N presents a high COF which exhibited the lowest wear rate. These can be explained by the existence of oxygen in the wear track. Moreover, low elastic strain to failure and mixing of ductile nanocrystalline Cu with TiAlN phase contributed to the high friction coefficient. Thus, the addition of Cu induced the formation of self-lubricant layer that improved the tribological behaviour of the film [66,67,68]. From Fig. 6, we can clearly notice that Ti(Al, Zr)N coating has the lowest CoF value of 0.29. This can be explained by the fact that a dense structure with a high surface roughness makes smaller contact areas between the film and the ball counterpart. Moreover, the presence of Zr induces a stable formation of the hard ZrN phase with good tribological performance, which is very useful for surface engineering process [2, 63, 66].
Surface features often change over the lifetime of the system. In addition, surface morphology and roughness can be modified due to the removal or displacement of part of the film material during the wear test. SEM images and 2D profiles of the wear tracks are presented to assess the morphological properties of the worn surfaces of films and balls (Figs. 8 and 9). To further explore the wear mechanisms of all coatings and balls, wear rates were also investigated (Fig. 7). As shown in Fig. 7a, the wear rate of Ti(Al)N, Ti(Al,Cu)N and Ti(Al, Zr)N coatings exhibited an increasing trend with applied load from 1 to 5 N. Moreover, as illustrated in Fig. 7b, the results showed that the ball wear rates have a same tendency to increasing with increasing the applied load. Whatever the applied load, it is showed that the addition of Zr to Ti(Al)N film has an important effect on the improvement of the wear rate, due to the high hardness, dense structure and grain refinement.
The tribological behaviour of three coatings tested under 1 N load was different, and various wear modes were observed. As can be seen from Fig. 8a, the (EDS, WDS) analysis performed on the wear tracks showed the presence of higher iron and oxygen contents suggesting the presence of some oxides. On the other hand, the formation of high wear particles on the surface of the Ti(Al)N coating produced severe scratch, resulting in poor frictional properties. Therefore, the worn zone on the 100Cr6 ball used against Ti(Al)N coating had the highest diameter of 590 μm with more debris on its surface as a result of material transfer between the ball and the coating (Fig. 9a). This debris in tribological contact raises the friction coefficient and wear rate of the coating.
The Ti(Al, Cu)N coating had worn track width and depth of 0.37 and 0.74 mm, respectively (Fig. 8b). The film delaminated on the inner side of the wear track, which was evident from the surface grooves on the worn surface with large amount of adhesive debris that formed micro-cracks and pits. The addition of Cu to the Ti(Al)N coating can inhibit crack propagation by preventing the removal of large amounts of film with each pass over the surface and thereby helps to reduce wear. The 100Cr6 steel ball rubbed against the Ti(Al, Cu)N coating showed a significant amount of film transfer with a high wear rate (8.1 × 10−5mm3/Nm and 5.9 × 10−3mm3/Nm) for coating and ball, respectively (Fig. 7). The majority of the transferred film appeared at the front edges of the contact area when the ball slid forward and, similarly, at the back of the contact area as the ball slid in the reverse direction. The formation of the transferred film explains the slight decrease in wear rate observed in the cross-sectional profiles of the wear tracks in the presence of more iron and oxygen in the worn surface that detected by EDS and WDS analysis.
Ti(Al, Zr)N coating showed some adhesive debris, but no severe wear line was observed. Similarity, the 100Cr6 steel ball showed some debris with no surface abrasion (Figs. 8c and 9c). From the EDS elemental analysis was noticed that the worn track was free of iron and oxygen, unlike Ti(Al, Cu)N coating. The depth, width, and rate of the wear track on the Ti(Al, Zr)N coating were 0.32 μm, 0.601 mm and 4.2 × 10−5mm3/Nm, respectively. This indicates a positive effect of Zr addition to improve the tribological performance of the Ti(Al)N coating. The good tribological performance of the Ti(Al, Zr)N coating can be related to its good hardness, high roughness, and high H/E and H3/E2 ratios. The high values of these coefficients confirm the high resistance of the film to elasto-plastic deformation, which leads to a good enhancement in its wear resistance.
Antibacterial properties
Figure 10 shows the bactericidal response of Ti(Al)N and TiAl(Cu, Zr)N nanocomposite coatings using gram-positive and gram-negative bacteria (S. aureus, B. subtilis, P. aeruginosa and E. coli). The coating behaviour was investigated in terms of inhibition of colony growth of these microorganisms. The TiAlN coating presented moderate activity against all the four used bacteria. The inhibition zones sizes were found in the 3–11 mm range. Similar results have been reported by Mejía et al. [47] where Al-doped TaN films showed low inactivation against S. aureus bacteria.
Ti(Al, Cu)N and Ti(Al, Zr)N nanocomposite coatings showed significant antibacterial activity against all bacteria, and the inhibition zones were significantly larger compared to Ti(Al)N as shown in Fig. 10. This could be explained by the surface roughness, grain size and shape of TiAl(Cu, Zr)N coatings. On the other hand, the intrinsic factors of nanoparticles exhibiting antibacterial activity are the elemental concentration and surface morphology (smaller size has greater bactericidal activity, especially if it is < 30 nm), which can promote redox reactions intracellular and extracellular, leading to cell death [47, 60]. Salhi et al. [2] found that the addition of Zr to TiN coating enhanced the surface roughness, modified the chemical composition and affected the bacterial adhesion. Liu et al. [69] reported that Ti-Cu alloy exhibited antibacterial properties against E. coli and S. aureus. Ti(Al)N containing copper or zirconium with low roughness, fine grain size and few lattice defects had good antibacterial properties (Fig. 11). The best antibacterial behaviour was obtained with the Ti(Al, Cu)N coating, indicating a large copper ion release, especially with E. coli and S. aureus. This behaviour is due to the bactericidal effect exerted by copper ions, which migrate from the nanocomposite into the culture medium. They cause damage to the cell membrane and alter enzymes, leading to a loss of cytoplasmic content, generating reactive oxygen species [70, 71].
Conclusion
TiAl(Cu, Zr)N coatings were deposited by magnetron sputtering on 316L stainless steel substrates. The effects of Zr and Cu on the structure, tribo-mechanical properties and biomedical behaviour of Ti(Al)N coatings were investigated.
The preferred orientation of TiAlN coating changed from (111) to (200) with Cu and Zr addition. Ti(Al, Zr)N and Ti(Al, Cu)N coatings exhibited compact columnar structure with globular and lamellar shape; however, the Ti(Al)N displayed a rough columnar structure with pyramidal-like form.
Zr addition was found more effective and suitable than Cu that led to enhance the hardness, the elastic modulus and the resistance of Ti(Al)N coating. The adhesion strength of Ti(Al)N coatings was obviously enhanced by Zr addition due to formation of ZrN phase.
TiAl(Cu, Zr)N nanocomposite coatings revealed high wear resistance and better bacterial inactivation behaviour. The results obtained in this study support the possibility of extending the service life of AISI 316L biomedical stainless steel by using a Ti(Al, Zr)N hard protective coating.
References
Lo KH, Shek CH, Lai JKL (2009) Recent developments in stainless steels. Mater Sci Eng R Rep 65(4):39–104
Salhi F, Aissani L, Falah M, Chadli A, Belgroune A, Nouveau C, Obrosov A, Abdul Samad M, Alhussein A (2021) Experimental investigation of structural, wetting, mechanical and Tribological properties of TiZrN thin films deposited by magnetron sputtering. Surf Interfaces 27:101519
Seale NS (2002) The use of stainless steel crowns. Pediatr Dent 24(5):501–505
Yang WJ, Zhang M, Zhao YH, Shen ML, Lei H, Xu L, Xiao JQ, Gong J, Yub BH, Sun C (2016) Enhancement of mechanical property and corrosion resistance of 316L stainless steels by low temperature arc plasma nitriding. Surf Coat Technol 298:64–72
Subramanian B, Umamaheswari G, Jayachandran M (2007) Properties and corrosion behaviour of reactive magnetron sputtered TiAlN coatings on AISI 316L SS in simulated bodily fluid. Corros Eng Sci Technol 42(4):349–355
Kayali Y (2014) The corrosion and wear behavior of TiN and TiAlN coated AISI 316 L stainless steel. Prot Met Phys Chem Surf 50(3):412–419
Xi Y, Bai Y, Gao K, Pang X, Yang H, Yan L, Volinsky A-A (2018) Residual stress and microstructure effects on mechanical, tribological and electrical properties of TiN coatings on 304 stainless steel. Ceram Int 44:15851–15858
Kumar TS, Prabu SB, Manivasagam G, Padmanabhan KA (2014) Comparison of TiAlN, AlCrN, and AlCrN/TiAlN coatings for cutting-tool applications. Int J Miner Metall Mater 21(8):796–805
Chen JT, Wang J, Zhang F, Zhang GA, Fan XY, Wu ZG, Yana PX (2009) Characterization and temperature controlling property of TiAlN coatings deposited by reactive magnetron co-sputtering. J Alloy Compd 472:91–96
Jinlong L, Rui W, Yongxin W, Liping W (2018) Anti-oxidant mechanism of TiAlN/SiN decorative films on borosilicate glass by magnetron sputtering. Int J Adv Manuf Technol 96(5):1563–1569
Yi P, Peng L, Huang J (2016) Multilayered TiAlN films on Ti6Al4V alloy for biomedical applications by closed field unbalanced magnetron sputter ion plating process. Mater Sci Eng C Mater Biol Appl 59:669–676
Shum PW, Li KY, Zhou ZF, Shen YG (2004) Structural and mechanical properties of titanium–aluminium–nitride films deposited by reactive close-field unbalanced magnetron sputtering. Surf Coat Technol 185(2):245–253
Aihua L, Jianxin D, Haibing C, Yangyang C, Jun Z (2012) Friction and wear properties of TiN, TiAlN, AlTiN and CrAlN PVD nitride coatings. Int J Refract Metal Hard Mater 31:82–88
Ramadoss R, Kumar N, Pandian R, Dash S, Ravindran TR, Arivuoli D, Tyagi AK (2013) Tribological properties and deformation mechanism of TiAlN coating sliding with various counterbodies. Tribol Int 66:143–149
Derflinger V, Schütze A, Ante M (2006) Mechanical and structural properties of various alloyed TiAlN-based hard coatings. Surf Coat Technol 200(16–17):4693–4700
Yang Q, Zhao LR, Zeng XT (2004) Erosion resistance performance of magnetron sputtering deposited TiAlN coatings. Surf Coat Technol 188–189:168–173
Braic M, Balaceanu M, Braic V, Vladescu A, Pavelescu G, Albulescu M (2005) “Synthesis and characterization of TiN, TiAIN and TiN/TiAIN biocompatible coatings. Surf Coat Technol 200(1):1014–1017
Twu MJ, Hu CC, Liu DW, Hsu CY, Kuo CG (2016) Effects of TiN, CrN and TiAlN coatings using reactive sputtering on the fatigue behaviour of AA2024 and medium carbon steel specimens. J Exp Nanosci 11(7):581–592
Subramanian B, Ashok K, Kuppusami P, Sanjeeviraja C, Jayachandran M (2008) Characterization of reactive DC magnetron sputtered TiAlN thin films. Cryst Res Technol 43(10):1078–1082
Leyendecker T, Lemmer O, Esser S, Ebberink J (1991) The development of the PVD coating TiAlN as a commercial coating for cutting tools. Surf Coat Technol 48(2):175–178
Chen L, Paulitsch J, Du Y, Mayrhofer PH (2012) Thermal stability and oxidation resistance of TiAlN coatings. Surf Coat Technol 206(11–12):2954–2960
Pei F, Liu HJ, Chen L, Xu YX, Du Y (2019) Improved properties of TiAlN coating by combined Si-addition and multilayer architecture. J Alloy Compd 790:909–916
Hollerweger R, Paulitsch J, Arndt M, Rachbauer R, Polcik P, Primig S, Mayrhofer PH (2014) Origin of high temperature oxidation resistance of TiAlTaN coatings. Surf Coat Technol 257:78–86
Tönshoff K, Karpuschewski B, Mohlfeld A, Leyendecker T, Erkens G, Fuß HG, Wenke R (1998) Performance of oxygen-rich TiAlON coatings in dry cutting applications. Surf Coat Technol 108–109:535–542
Zhang X, Jiang J, Yuqiao Z, Lin J, Wang F, Moore JJ (2008) Effect of carbon on TiAlCN coatings deposited by reactive magnetron sputtering. Surf Coat Technol 203(5):594–597
Wang CF, Ou SF, Chiou SY (2014) Microstructures of TiN, TiAlN and TiAlVN coatings on AISI M2 steel deposited by magnetron reactive sputtering. Transactions Nonferrous Metals Soc China 24(8):2559–2565
Volkhonsky IO, Blinkov IV, Belov DS (2020) The effect of the metal phase on the compressive and tensile stresses reduction in the superhard nitride coatings. Coatings 10(8):798
Sert Y, Küçükömeroğlu T, Efeoğlu İ (2020) Quantification of the effects of coating parameters on the properties of TiAlZrN coatings. Brill Eng 4:1–8
Moritz Y, Kainz C, Tkadletz M, Czettl C, Pohler M, Schalk N (2014) Microstructure and mechanical properties of nanostructured Ti–Al–Si–N coatings deposited by magnetron sputtering. Surf Coat Technol 241:105–111
Tillmann W, Grisales D, Stangier D, Thomann C, Debus J, Nienhaus A, Apel D (2021) Residual stresses and tribomechanical behaviour of TiAlN and TiAlCN monolayer and multilayer coatings by DCMS and HiPIMS. Surf Coat Technol 406:126664
Mani SP, Rikhari B, Agilan P, Rajendran N (2018) Evaluation of the corrosion behavior of a TiN-coated 316L SS bipolar plate using dynamic electrochemical impedance spectroscopy. New J Chem 42:14394–14409
Holzwarth U, Gibson N (2011) The scherrer equation versus the’’Debye-Scherrer equation’. Nat Nanotechnol 6:534–534
GG Stoney (1909) The tension of metallic films deposited by electrolysis, Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 82,172–175
Kossman S, Coorevits T, Iost A, Chicot D (2017) A new approach of the Oliver and Pharr model to fit the unloading curve from instrumented indentation testing. J Mater Res 32:2230–2240
Aysa NH and Salman HD (2016) Antibacterial activity of modifiedzinc oxide nanoparticles against Pseudomonas aeruginosa isolate of burn infections, World Scientific News, 1–14
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single diskmethod. Am J Clin Pathol 45:493–496
Abadias G, Tse YY, Guerin Ph, Pelosin V (2006) Interdependence between stress, preferred orientation, and surface morphology of nanocrystalline TiN thin films deposited by dual ion beam sputtering. J Appl Phys 99(113519):1–13
Musil J, Hruby H (2000) Superhard nanocomposite Ti1−xAlxN films prepared by magnetron sputtering. Thin Solid Films 365(1):104–109
Aissani L, Alhussein A, Nouveau C, Ghelani L, Zaabat M (2019) Influence of film thickness and Ar-N2 plasma gas on the structure and performance of sputtered vanadium nitride coatings. Surf Coat Technol 378(25):124948
Hongbo J, Dian Y, Lihua X, Bin Z, Yaoxiang G, Shao H-T, Ren L, Chengzhong LD, Hongfei Z, Hongzhao M (2019) Crystal structure and tribological properties of Zr-Al-Mo-N composite films deposited by magnetron sputtering. Mater Chem Phys 230:347–354
Chen L, Moser M, Du Y, Mayrhofer PH (2009) Compositional and structural evolution of sputtered Ti-Al-N. Thin Solid Film 517:6635–6641
Chen L, Du Y, Wang SQ, Li J (2007) A comparative research on physical and mechanical properties of (Ti, Al)N and (Cr, Al)N PVD coatings with high Al content. Int J Refract Met Hard Mater 25(5–6):400–404
Zou C, Zhang J, Xie W, Shao L, Fu D (2011) Characterization and mechanical properties of Ti–Al–Cr–N nanocomposite coatings deposited by closed field unbalanced middle frequency magnetron sputtering. Jpn J Appl Phys 50(12R):125806
Aissani L, Fellah M, Belgroune A, Obrosov A, Samad MA, Alhussein A (2021) Effect of O2 flow rate on the structure, wettability and tribo-mechanical behaviour of Zr-ON thin films. Surf Interfaces 26:101441
Bagcivan N, Bobzin KK, Theis S (2013) Cr1-xAlxN: a comparison of direct current, middle frequency pulsed and high power pulsed magnetron sputtering for injection molding components. Thin Solid Films 528:180–186
Shimokawa T, Nakatani A, Kitagawa H (2005) Grain-size dependence of the relationship between intergranular and intragranular deformation of nanocrystalline Al by molecular dynamics simulations. Phys Rev B. https://doi.org/10.1103/physrevb.71.224110
Gangopadhyay S, Acharya R, Chattopadhyay AK, Paul SS (2010) Effect of substrate bias voltage on structural and mechanical properties of pulsed DC magnetron sputtered TiN–MoSx composite coatings. Vacuum 84:843–850
Bagcivan N, Bobzin K, Theiß S (2013) (Cr1−xAlx)N: a comparison of direct current, middle frequency pulsed and high power pulsed magnetron sputtering for injection molding components. Thin Solid Film 528:180–186
Chenkai L, Hongbo J, Junhua X, Lihua Y, Zitong Z, Yaoxiang G, Yong Z (2020) Influence of copper on the compositions, microstructure and room and elevated temperature tribological properties of the molybdenum nitride film. Surf Coat Technol 395:125811
Malygin GA (2007) Plasticity and strength of micro-and nano crystalline materials. Phys Solid State 49:1013–1033
Subramanian B, Ananthakumar R, Jayachandran M (2010) Microstructural, Mechanical and electrochemical corrosion properties of sputtered titanium aluminium nitride films for bio-implants. Vacuum 85:601–609
Yang B, Chen L, Xu YX, Peng YB, Fen JC, Du Y, Wu MJ (2013) Effect of Zr on structure and properties of Ti–Al–N coatings with varied bias. J Refract Met Hard Mater 38:81–86
Hollstein F, Wiedemann R, Scholz J (2003) Characteristics of PVD-coatings on AZ31hp magnesium alloys. Surf Coat Technol 162(1–2):261–268
Zhao SS, Du H, Hua WG, Gong J, Li JB, Suna C (2007) The depth distribution of residual stresses in (Ti, Al)N films: measurement and analysis. J Mater Res 22(10):2659–2662
Aissani L, Alhussein A, Nouveau C, Radjehi L, Lakdhar I, Zgheib E (2019) Evolution of microstructure, mechanical and tribological properties of vanadium carbonitride coatings sputtered at different nitrogen partial pressures. Surf Coat Technol 374:531–540
Zou C, Zhang J, Xie W, Shao L, Fu D (2011) Characterization and mechanical properties of Ti–Al–Cr–N nanocomposite coatings deposited by closed field unbalanced middle frequency magnetron sputtering. Jpn J Appl Phys 50(12):125806
Aissani L, Alhussein A, Ayad A, Nouveau C, Zgheib E, Belgroune A, Zaabat M, Barille R (2021) Relationship between structure, surface topography and tribo-mechanical behavior of Ti-N thin films elaborated at different N2 flow rates. Thin Solid Films 724:138598
Belov DS, Blinkov IV, Volkhonskii AO (2014) The effect of Cu and Ni on the nanostructure and properties of arc-PVD coatings based on titanium nitride. Surf Coat Technol 260:186–197
Sert Y, Kucukomeroglu T, Efeoglu I (2020) Investigating the structure, adhesion and tribological properties of Al and Zr-doped TiN coatings with various substrate bias voltages and working pressure. J Eng Tribol 235(6):1190–1202
Mejía HD, Echavarría AM, Bejarano GG (2019) Influence of Ag-Cu nanoparticles on the microstructural and bactericidal properties of TiAlN(Ag, Cu) coatings for medical applications deposited by Direct Current (DC) magnetron sputtering. Thin Solid Films 687:137460
Franková J, Pivodová V, Růžička F, Tománková K, Šafářová K, Vrbková J, Vrbková J, Ulrichová J (2013) Comparing biocompatibility of gingival fibroblasts and bacterial strains on a different modified titanium discs. J Biomed Mater Res A 101:2915–2924
Hussein MA, Abdul Samad M, Al-Aqeeli N (2015) Wear characteristics of metallic biomaterials: a review materials. Mater 8(5):2749–2768
Aissani L, Fellah M, Radjehi L, Nouveau C, Montagne A, Alhussein A (2019) Effect of annealing treatment on the microstructure, mechanical and tribological properties of chromium carbonitride coatings. Surf Coat Technol 359:403–413
Blau PJ (2005) On the nature of running-in. Tribol Int 38(11–12):1007–1012
Satyananda K, Sahu BB, Kousaka H, Han JG, Hori M (2020) Effect of substrate surface roughness on adhesion of titanium nitride coatings deposited by physical vapour deposition technique. IOP Conf Ser Mat Sci. Eng 981:1–9
Kara L, Özkan D, Yağci MB, Sulukan E, Sert Y, Sert TS (2019) Friction and wear behaviors of TiN coatings under dry and vacuum conditions. Tribol Trans 62:362–373
Vlasveld AC, Harris SG, Doyle ED, Lewis DB, Munz WD (2002) Characterisation and performance of partially filtered arc TiAlN coatings. Surf Coat Technol 149:217–223
Brunello G, Brun P, Gardin C, Ferroni L, Bressan E, Meneghello R, Zavan B, Sivolella S (2018) J Plos one 13(6):0199591
Amoroso PF, Adam RJ, Waters MGJ, Williams DW (2006) Titanium surface modification and its effect on the adherence of Porhyromonas gingivalis: an in vitro study. Clin Oral Implants Res 17:633–637
Liu J, Zhang X, Wang H, Li F, Li M, Yang K, Zhang E (2014) The antibacterial properties and biocompatibility of a Ti-Cu sintered alloy for biomedical application. Biomed Mater 9(025013):1–11
Andrés GM, Jofre J, Tijerina EP, Pomar SS, Sanhueza GS, Meléndrez MF (2021) Effect of titanium coated with 3 different types of copper nanoparticles in the oral biofilm formation. J Dent Health Oral Disord Ther 12(1):1–6
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aissani, L., Belgroune, A., Saoudi, A. et al. Tribo-mechanical performance and antibacterial activity in (Cu, Zr)-alloyed Ti(Al)N coatings synthesized by reactive magnetron sputtering. J Mater Sci 57, 19612–19630 (2022). https://doi.org/10.1007/s10853-022-07804-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07804-6