Abstract
Growth of bionanotechnology requires functional materials, which can lower the costs and can be modified to the specific reaction. Conjugations of nanoparticles and enzymes form efficient products of multi-material enzyme-nanocomplexes, which can be controlled from synthesis to application and can provide predicted results. Multimetallic nanooxides of ZnO–CuO, ZnO–MnO, ZnO–MnO–CuO were obtained by precipitation method with sonication, followed by microwave process. Superoxide dismutase (SOD) was immobilized on the surface of the nanoparticles. Obtained nanomaterial-enzyme complexes had antioxidant properties. Particles were characterised by XRD, SEM and TEM methods and ATR spectroscopy which proved enzyme-nanooxides conjugation. Scavenging activity of the materials was on average 85% in DPPH (2,2-diphenyl-1-picrylhydrazyl) method and 20 mg/l in TROLOX (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) concentration, where pure SOD enzyme presented around 90% and 10 mg/l activity, respectively. Conjugation of the highest antioxidant power is ZnO–MnO with SOD, however all three types of materials could be used in further applications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of substances of natural origin to carry out various catalysis processes or drug delivery influences the development of biotechnology and related fields, such as nanotechnology and biology. Currently, it is important to design multifunctional materials tailored to the needs of research, hence the multi-complexes of nanomaterials and biologically active substances or nanozymes—are very popular [1,2,3]. Nanozymes can mimic or fully replace natural enzymes, which are expensive catalysts [4]. Nanozymes fully replacing natural enzymes can be metal oxides such as Mn3O4 or metal nanoparticles as Pt and Cu, which can provide SOD-like activity for reducing reactive oxygen species [5, 6]. Furthermore, materials with antioxidant activity could be used for treatment and reducing negative results of oxidative stress in cells—such as cerium vanadate in neuronal cells [7]. Nanozymes and enzyme-nanocomplexes can be modified and adjusted to the reaction, they can serve as biosensors, catalysts and in many other biomedical uses [8, 9].
Lowering production costs, high stability or increasing the efficiency of particles are the main goals for new methods developed for obtaining nanocomplex materials [10]. Multi-material enzyme-nanocomplexes allow to combine the functions of several substances, control the size of the synthesized particles or enhance the properties of the final product [11]. As a result of using the immobilization techniques, the product should be stable and used repeatedly or delivered to a specific place in the body. These are the goals that can be achieved by nanozymes and multi-complexes, which makes them interesting topic of research.
The immobilization technique requires an appropriate selection of the method and matrix material. The matrix-enzyme combination reduces the impact of unfavourable conditions such as pH or reaction environmental characteristics, and also reduces the sensitivity of the peptide to the presence of inhibitors. Additionally, the multiple use of the enzyme reduces the final cost of biocatalysis [12,13,14]. Increasing the mechanical strength of the protein structure allows for extending the storage time of such catalytic system [15, 16] and the system can be easily separated from the reaction mixture [16, 17]. Immobilization by adsorption is a low-cost method, widely used due to its simplicity. Additionally, weak interactions such as hydrogen bonds and van der Waals forces do not interfere with enzyme structure, which allows to maintain high activity of the protein [18].
The objective of this study was to carry out the immobilization process of superoxide dismutase onto surfaces of oxide nanoparticles. Superoxide dismutase (SOD) is an enzyme with EC 1.15.1.1 classification number and belongs to the group of oxidoreductases. It uses superoxide as an acceptor in catalysed electron transfer reactions [19]. The superoxide radical is a by-product of aerobic respiration, phosphorylation or photosynthesis reactions. Superoxide dismutase breaks it into molecular oxygen and hydrogen peroxide with the help of the hydrogen cation [20]. As the result functional materials were obtained and their antioxidant properties were measured by inhibition of DPPH (2,2-diphenyl-1-picrylhydrazyl) radical and in FRAP (Ferric-reducing antioxidant power) assay. Additionally, stability of the obtained systems was determined during the study.
Materials and methods
Materials
Copper (II) sulphate pentahydrate CuSO4·5H2O (Sigma Aldrich), zinc (II) chloride ZnCl2 (Sigma Aldrich) and manganese (II) sulphate monohydrate MnSO4·H2O (Sigma Aldrich) were used as oxide nanoparticle precursors. Sodium hydroxide NaOH (Sigma Aldrich) was used as precipitating agent. The enzyme tested was superoxide dismutase expressed in E. Coli (Sigma Aldrich).
Synthesis of nanoparticles
The solution of ZnCl2 (3.5 M, V = 4.82 ml) was added to the Teflon vessel and while mixing an aqueous solution of NaOH (3 M, V = 14.52 ml) was added and a precipitating agent. Next, in the presence of ultrasounds (Hielscher UP400St sonicator) other solutions, namely CuSO4 (0.62 M, V = 5.69 ml) or MnSO4 (0.96 M, V = 6.00 ml) or both, were added and precipitated by an excess of NaOH. Sonication was performed for 5 minutes. Then, in MAGNUM II microwaves reactor at 180 °C for 10 min, dehydration of previously formed hydroxides was carried out to obtain ZnO–CuO, ZnO–MnO, ZnO–MnO–CuO particles. At first, zinc hydroxide particles were formed during the precipitation and later manganese hydroxide or copper hydroxide or both were formed in the presence of ultrasounds, forming multimetallic nanooxides after the microwave process. Afterwards, materials were filtered, washed and dried in 70 °C overnight. All materials were calcinated in 500 °C for 2h after drying.
Preparation of SOD immobilised on metal oxide nanoparticles
To 1 mg of nanoparticles, SOD solution was added in according ratio and filled with ultrapure water to 100 μl volume. Stock solution of SOD had concentration of 2 mg/ml, enzyme concentrations during immobilization were: 0.5 mg/ml; 1.0 mg/ml; 1.5 mg/ml. Each kind of nanoparticles was immobilized in each enzyme concentration, as a result there were 9 different catalytic systems. Immobilization of SOD was carried out for 120 minutes, in 25 °C. At the end of the process, particles were separated from the solution and the supernatant was examined spectrophotometrically to analyse the enzyme concentration by Pierce BCA (Bicinchoninic Acid) ThermoFisher kit.
Efficiency of the process was calculated basing on the protein concentration in the supernatant, from the equation:
where m0—mass of the SOD in primary solution (mg) used for immobilization, m1—mass of the SOD after immobilization (mg).
The amount of the enzyme adsorbed on the particles was determined as a ratio from equation:
where mnanoparticles—mass of metal oxide nanoparticles (g), V—volume of the enzyme solution, C0—concentration of primary enzyme solution, C1—concentration of enzyme solution after the immobilization
Materials characteristics
The UV–Vis spectroscopy was used to quantify the SOD concentration in the solutions. For the SOD determination BCA Pierce ThermoFisher kit was used and measurement was performed at 562 nm with Synergy2 TM Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). The test is a method based on copper ion reduction with bicinchoninic acid. The reagents were prepared right before the measurements, the reference curves were made using bovine albumine, samples were incubated in 37 °C for 30 min before the measurement.
The quality analysis of nanoparticles crystallites was determined by X-ray diffractometry (XRD) method using Philips X’Pert Camera diffractometer with PW 1752/00 CuKa monochromator in the range of angles 2θ, from 10 to 60°. Additionally, the size of crystallites was calculated from Scherrer’s equation:
where d—average size of crystallites, FWHM—peak width at half of its height, proportional to the size of the crystallite, K—Scherrer’s constant depending on the size, λ—X-ray wavelength, θ—angle formed by radiation with the atomic plane. The constant K was selected based on SEM microphotography.
The morphologies of the obtained metal oxide complexes were discovered through scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS, FEI QUANTA 400 FEG ESEM/EDAX PEGASUS X4M) and transmittance electron microscopy (TEM). For determination of the specific functional groups in the metal oxide nanocomplexes, Attenuated Total Reflectance spectroscopy (ATR) was performed in the range from 4000 to 300 cm−1.
Antioxidant properties of nanooxide particles
The aim of the study was obtaining active enzyme-nanomaterial conjugation which can be applicable as antioxidant functional material. The activity of the obtained enzyme-nanocomplexes was determined by methods with 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) reagents.
In the first method, violet DPPH radical reacts with the protein and forms yellow solution. Nitrogen radical in the structure of DPPH is connected with R-group coming from the protein. The activity of this scavenging radical is presented as a percentage of inhibition of the radical. Concentration of the solution was adjusted to present absorbance of 1 as the zero sample. Later the sample was added to the DPPH reagent 3 ml and measured at 517 nm.
The blank sample was performed the same method but 40 μl of ethanol was added to DPPH reagent. The activity was described as percentage of immobilized DPPH radical from equation:
where A0—absorbance of DPPH solution, A—absorbae of sample
The FRAP method measures the reduction of Fe3+ complexes to the Fe2+ complexes in an acidic medium. After reaction with an antioxidant, Fe2+ ion is formed and complexed with solvent to form blue coloured product measured spectrophotometrically. The FRAP reagent was prepared from acetate buffer, TPTZ (2,4,6-tripyridyl-s-tri-divine) and ferric chloride. The reagent was incubated for 20 minutes before addition. Later the sample was added to 2 ml of FRAP reagent, incubated for 30 minutes and then measured at 593nm. The calibration curve was based on Trolox reaction with FRAP reagent.
Studies on stability of immobilized nanosystems
After the immobilization process, pellets of nanoparticles with immobilized SOD enzyme were kept in 10 °C conditions. After 21 days and 28 days, 0.4 ml of distilled water was added to the pellets, then centrifuged and supernatant was separated from the particles again. Concentration of proteins in the supernatant was determined with BCA kit, in the same method as previously. Later, stability was presented in the same way as sorption efficiency in (%).
Statistical analysis
All samples were performed in triplicates. For the antioxidant properties results, analysis of variance (ANOVA) was calculated for F-test distribution (F (3,8)) and p < 0.05 as statistically significant. Additionally post-hoc test—Sheffe was chosen for investigating differences between samples.
Results and discussion
Immobilisation of SOD on metal oxide complexed nanoparticles
Obtained nanoparticles of ZnO–MnO (A), ZnO–CuO (B) and ZnO–MnO–CuO (C) were used for immobilisation with different primary concentrations of SOD enzyme: 0.5 mg/ml (1), 1.0 mg/ml (2) and 1.5 mg/ml (3). Figure 1 presents the best conjugations of enzyme on particles with calculated sorption capacity with letter Q (mg/g) and efficiency of the immobilization process (%). From all analysed materials, three most effective materials were chosen for further antioxidant analysis and they are highlighted by colour and number tag. To obtain the highest Q, it is desirable to use enzyme in concentration 1.5 mg/ml. The amount of absorbed protein on the particles increased with the concentration.
Physicochemical characteristics of the nanoparticles and the enzyme-nanocomplexes
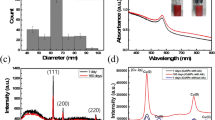
Following diffractograms present metal oxide nanoparticles analysed by XRD. Figure 2 shows ZnO–MnO, ZnO–CuO and ZnO–MnO–CuO particles.
All diffractograms show characteristic ZnO peaks—three at 30°–36° with different intensities: first of lower intensity, second the smallest, third at 36° the most intense and two at 46° and 56° [21]. For ZnO–CuO peak at 36° has higher intensity than the same one for ZnO–MnO which can be related to copper present in the sample [22, 23]. Additionally, at 35.5° and 38° two smaller peaks can be observed in ZnO–CuO sample and they confirm copper oxide present in the sample [22]. In ZnO–MnO–CuO, zinc peaks are less intense or less visible, however diffractogram still represents characteristic peaks and confirms presence of the nanooxide particles.
Based on diffractograms and Scherrer’s equation the size of crystallites and composition were determined. Table 1 presents sizes of each phase for all types of particles. Obtained diffractograms and calculated particle sizes are similar to found in literature. Doan et al. obtained ZnO–CuO composites via simple percipitation, sonification and later stirring for 24 h and drying and calcination. The autors identified the two crystallite phases and resulted in sizes on average 40 nm [24]. Khalaji et al. prepared single CuO nanoparticles via calcination where size of the single particle was around 70 nm [25]. In the study, the nanocomposites were in the similar size despite addition of another nanooxide.
Additionally, obtained materials were analysed in SEM-EDS method presented in Figure 3. Structures of each complex are visible on the images. EDS analysis confirmed presence of each element, namely MnO, CuO, ZnO for obtained oxide materials. EDS analysis was performed accordingly for each area showed on SEM images.
Furtherly, morphology of the samples was determined by TEM method (Figure 4). Nanooxide particles form sphere-like and cubic shapes clearly visible on microphotographs. Similarly, to SEM, single particles are visible and despite connections, particles do not form aggregates. Figure 4 presents nanooxides particles (left) and enzyme-nanocomplexes (right). TEM analysis proved that there were no changes in microstructure of nanooxide particles after the immobilization of SOD enzyme.
Ateka et al. obtained ZnO–CuO–MnO particles in sizes less than 100 nm. They used precipitation method and drying for 24 h and calcination at 300 °C for 10 h [26]. Khalil et al. presented the results for obtaining ZnO nanoparticles from curcumin complex by thermal decomposition. TEM image analysed by the authors was similar to the images in the study, despite differences of addition of other oxides. ZnO particles sizes were bigger than ones obtained in the study (117 nm and 46 nm, accordingly) [27].
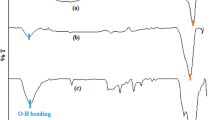
The samples of nanooxides particles and nanooxides particles immobilized with the SOD enzyme were examined by ATR (Fig. 5). The bands between 400 to 700 cm−1 are associated to metal-oxide bands in each type of particles. In 1450–1800 cm−1, the bands appear in immobilized nanoparticles spectra and can be associated with amide bond present in protein structures. The bands at 3500-3800 cm-1 are connected to –OH bond, present in protein structures and between metal oxide and enzyme particles.
Stability of nanooxide particles immobilized with SOD enzyme
Figure 6 presents graphical data on stability of the nanosystems as efficiency of sorption in [%] for each enzyme concentration (1: 0.5 mg/ml, 2: 1.0 mg/ml, 3: 1.5 mg/ml) and type of nanooxide (A, B, C). All complexes show instability, however sample 1C notes the highest values of all materials. Low stability of the samples can be connected to storage conditions and it suggest changes to the method of this analysis. The results can find proof in other research articles where Danial and Alkhalaf prepared SOD immobilized with alginate gel beads and the stability of the particles was less than 35 days. However, the immobilized materials are different than in the study, which makes it difficult to fully compare the two results [28].
The antioxidant properties SOD on nanooxide particles
The antioxidant properties were confirmed by two methods DPPH and FRAP. The activity of the DPPH scavenging radical is presented as a percentage of inhibition of the radical. Figure 7 presents the activity for obtained nanomaterials and pure SOD enzyme, which were chosen as stated in section 3a. Lower scavenging property of the two materials can be the result of immobilization, where active sites of SOD are not available for the substrate. However, the results are not very different from pure enzyme. Additionally, nanooxide particles can provide different properties such as antimicrobial. For the obtained results, analysis of variance (ANOVA) was calculated (F (3,8) = 1.3272, p = 0.3317). The post-hoc test—Sheffe proved that there was no significant difference between samples.
Saringer et al. immobilized SOD on titania nanosheets with poly(diallyl dimethylammonium chloride) and poly(styrene sulfonate) and peroxidase in different conjugations. Pure SOD presented around 86% of inhibiting activity, while other conjugations with presented materials resulted in activity between 80 and 93%. The results in the study are in accordance with these presented by the authors [29]. The same authors previously obtained similar results just for SOD immobilized on titania nanosheets with poly(diallyl dimethylammonium chloride) [30].
Activity of the FRAP method is presented in Fig. 8 as TROLOX concentration in mg/l. For the obtained results, analysis of variance (ANOVA) was calculated (F (3,8) =601,52, p < 0.001). Type of the material turned out to be significant factor influencing the FRAP analysis. The post-hoc test—Sheffe proved that only effects for materials ZnO–CuO and ZnO–CuO–MnO are not statistically different (p > 0.05). The FRAP method also suggests that ZnO–MnO+SOD nanomaterial shows the highest antioxidant potential. However, comparing antioxidant activity of nanomaterials to pure SOD, it can be observed that nanomaterials are more effective. ZnO-MnO nanooxide material can result in better results, because of probable positive effect on SOD enzyme. Superoxide dismutase used in this research was of manganese type, where Mn is the enzymatic cofactor [31]. The DPPH and FRAP results can be compared to other literature, showing slightly lower values [28, 30, 32]. Additionally, immobilization for some materials, also lowered activity results for SOD [28].
Conclusion
In this study, obtained multimetallic complexes are nanomaterials, which was confirmed by SEM-EDS, TEM and XRD methods. Diffractometry confirmed the composition of the nanooxides and phase sizes, while TEM and SEM microphotographs presented shape and morphology of the surface.
The most favourable parameters for sorption of SOD on ZnO–CuO, ZnO–MnO and ZnO–MnO–CuO were: temperature 20 °C, concentration of enzyme solution 1.5 mg/ml, sorption time 120 min. Immobilisation was efficient process resulting in: av. 50% sorption of the enzyme on each type of particles and over 50 mg of conjugated enzyme per gram of particles for the most preferred conditions. The immobilisation was confirmed by FT-IR spectroscopy.
In the stability study, obtained systems showed comparable stability to other studies. Method of testing or upgrades to the sorption process should be done for desired stability effect for catalytic systems.
Antioxidant activity was confirmed by two methods, which confirmed activity of immobilised enzyme on each nanooxide. All three particle types could be useful in further application. There seems to be a correlation between material and enzyme activity, which was the highest for ZnO–MnO nanoparticles, probably in line of the type of the SOD enzyme metallic centre.
References
Tipton K, McDonald A (2018) A brief guide to enzyme nomenclature and classification. In: International union of biochemistry and molecular biology. https://iubmb.org/wp-content/uploads/sites/10116/2018/11/A-Brief-Guide-to-Enzyme-Classification-and-Nomenclature-rev.pdf. Accessed 7 Mar, 2021
Qiao P, Zou S, Xu S et al (2014) A general synthesis strategy of multi-metallic nanoparticles within mesoporous titania via in situ photo-deposition. J Mater Chem A 2:17321–17328. https://doi.org/10.1039/c4ta02970d
Yang P, Tao J, Chen F et al (2021) Multienzyme-mimic ultrafine alloyed nanoparticles in metal organic frameworks for enhanced chemodynamic therapy. Small 17:2005865. https://doi.org/10.1002/SMLL.202005865
Huang X, Zhang S, Tang Y et al (2021) Advances in metal–organic framework-based nanozymes and their applications. Coord Chem Rev 449:214–216. https://doi.org/10.1016/J.CCR.2021.214216
Liu Y, Zhang Y, Liu Q et al (2021) In vitro measurement of superoxide dismutase-like nanozyme activity: a comparative study. Analyst 146:1872–1879. https://doi.org/10.1039/d0an02164d
Yang J, Zhang R, Zhao H et al (2022) Bioinspired copper single-atom nanozyme as a superoxide dismutase-like antioxidant for sepsis treatment. Exploration. https://doi.org/10.1002/EXP.20210267
Singh N, NaveenKumar SK, Geethika M, Mugesh G (2021) A cerium vanadate nanozyme with specific superoxide dismutase activity regulates mitochondrial function and ATP synthesis in neuronal cells. Angew Chem Int Ed 60:3121–3130. https://doi.org/10.1002/ANIE.202011711
Sharma G, Kumar A, Sharma S et al (2019) Novel development of nanoparticles to bimetallic nanoparticles and their composites: a review. J King Saud Univ Sci 31:257–269
Chen K, Sun S, Wang J, Zhang XD (2021) Catalytic nanozymes for central nervous system disease. Coord Chem Rev 432:213751–213760. https://doi.org/10.1016/J.CCR.2020.213751
Tang G, He J, Liu J et al (2021) Nanozyme for tumor therapy: surface modification matters. Explor 1:75–89. https://doi.org/10.1002/EXP.20210005
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235. https://doi.org/10.1039/c3cs60075k
Sokołowska K, Konieczna-Molenda A, Witek E (2015) Cellulase immobilized on copolymers based on N-vinylformamide: Influence of immobilization on the catalytic activity of the enzyme. Chemik 69:451–454
Bolibok P (2016) Enzyme immobilization on carriers as a way of directed modification of biocatalysator properties Immobilizacja enzymów na nośnikach sposobem na ukierunkowaną modyfikację właściwości biokatalizatorów. Przemysł Chemiczny 1:124–128. https://doi.org/10.15199/62.2016.11.22
Darwesh OM, Matter IA, Eida MF (2019) Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J Environ Chem Eng 7:102805–102810. https://doi.org/10.1016/j.jece.2018.11.049
Meryam Sardar RA (2015) Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Biochem Anal Biochem. https://doi.org/10.4172/2161-1009.1000178
Zdarta JŁ (2017) Immobilizacja enzymów na wybranych nośnikach organicznych i nieorganicznych
Chałupka Z (2016) Immobilizowanie alfa-amylazy i celulazy w zastosowaniach praktycznych. “Wiadomości Chemiczne” Czasopismo Polskiego Towarzystwa Chemicznego 70:369–389
Kaleem I, Rasool A, Lv B et al (2017) Immobilization of purified β-glucuronidase on ZnO nanoparticles for efficient biotransformation of glycyrrhizin in ionic liquid/buffer biphasic system. Chem Eng Sci 162:332–340. https://doi.org/10.1016/J.CES.2016.12.074
Zhao H, Zhang R, Yan X, Fan K (2021) Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J Mater Chem B 9:6939–6957. https://doi.org/10.1039/D1TB00720C
BRENDA Information on EC 1.15.1.1-superoxide dismutase. https://www.brenda-enzymes.org/enzyme.php?ecno=1.15.1.1. Accessed 8 Mar 2021
van Heerden JL, Swanepoel R (1997) XRD analysis of ZnO thin films prepared by spray pyrolysis. Thin Solid Films 299:72–77. https://doi.org/10.1016/S0040-6090(96)09281-4
Ashokan S, Ponnuswamy V, Jayamurugan P, Rao YVS (2015) Fabrication and characterization of CuO nanoparticles: its humidity sensor application. South Asian J Eng Technol 1:11–23. https://doi.org/10.1016/J.JALLCOM.2015.05.088
Elbasuney S (2018) Novel colloidal nanothermite particles (MnO2/Al) for advanced highly energetic systems. J Inorg Organomet Polym Mater 28:1793–1800. https://doi.org/10.1007/s10904-018-0823-x
Doan TLH, Kim J-Y, Lee J-H et al (2021) Facile synthesis of metal-organic framework-derived ZnO/CuO nanocomposites for highly sensitive and selective H2S gas sensing. Sens Actuat B Chem 349:130741–130750. https://doi.org/10.1016/j.snb.2021.130741
Khalaji AD, Pazhand Z, Kiani K et al (2020) CuO nanoparticles: preparation, characterization, optical properties, and antibacterial activities. J Mater Sci Mater Electr 31:11949–11954. https://doi.org/10.1007/S10854-020-03749-1
Ateka A, Sierra I, Ereña J et al (2016) Performance of CuO–ZnO–ZrO2 and CuO–ZnO–MnO as metallic functions and SAPO-18 as acid function of the catalyst for the synthesis of DME co-feeding CO2. Fuel Process Technol 152:34–45. https://doi.org/10.1016/J.FUPROC.2016.05.041
Khalil MI, Al-Qunaibit MM, Al-zahem AM, Labis JP (2014) Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex. Arab J Chem 7:1178–1184. https://doi.org/10.1016/J.ARABJC.2013.10.025
Danial EN, Alkhalaf MI (2020) Co-immobilisation of superoxide dismutase and catalase using an in vitro encapsulation protocol. J King Saud Univ Sci 32:2489–2494. https://doi.org/10.1016/j.jksus.2020.04.003
Sáringer S, Rouster P, Szilagyi I (2021) Co-immobilization of antioxidant enzymes on titania nanosheets for reduction of oxidative stress in colloid systems. J Colloid Interface Sci 590:28–37. https://doi.org/10.1016/J.JCIS.2021.01.012
Rouster P, Pavlovic M, Szilagyi I (2018) Immobilization of superoxide dismutase on polyelectrolyte-functionalized titania nanosheets. ChemBioChem 19:404–410. https://doi.org/10.1002/cbic.201700502
Chouchani ET, Pell VR, James AM et al (2016) A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab 23:254–263. https://doi.org/10.1016/J.CMET.2015.12.009
Bela RT, Benjamin ET, Christina LN (2017) Antioxidant capacity and superoxide dismutase activity in adrenoleukodystrophy. JAMA Neurol 74:519–524. https://doi.org/10.1001/JAMANEUROL.2016.5715
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matysik, J., Długosz, O., Loureiro, J. et al. Multioxide-superoxide dismutase enzyme-nanocomplexes and their antioxidant activity. J Mater Sci 57, 15954–15966 (2022). https://doi.org/10.1007/s10853-022-07620-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07620-y