Abstract
Particle number densities are a crucial parameter in the microstructure engineering of microalloyed steels. We introduce a new method to determine nanoscale precipitate number densities of macroscopic samples that is based on the matrix dissolution technique (MDT) and combine it with atom probe tomography (APT). APT counts precipitates in microscopic samples of niobium and niobium-titanium microalloyed steels. The new method uses MDT combined with analytical ultracentrifugation (AUC) of extracted precipitates, inductively coupled plasma–optical emission spectrometry, and APT. We compare the precipitate number density ranges from APT of 137.81 to 193.56 × 1021 m−3 for the niobium steel and 104.90 to 129.62 × 1021 m−3 for the niobium-titanium steel to the values from MDT of 2.08 × 1021 m−3 and 2.48 × 1021 m−3. We find that systematic errors due to undesired particle loss during extraction and statistical uncertainties due to the small APT volumes explain the differences. The size ranges of precipitates that can be detected via APT and AUC are investigated by comparison of the obtained precipitate size distributions with transmission electron microscopy analyses of carbon extraction replicas. The methods provide overlapping resulting ranges. MDT probes very large numbers of small particles but is limited by errors due to particle etching, while APT can detect particles with diameters below 10 nm but is limited by small-number statistics. The combination of APT and MDT provides comprehensive data which allows for an improved understanding of the interrelation between thermo-mechanical controlled processing parameters, precipitate number densities, and resulting mechanical-technological material properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalloyed steels find application in structural part production across numerous industrial sectors such as offshore and pipeline industry or the automotive sector. Their low carbon content ensures good weldability while displaying compelling mechanical properties compared to mild steel. Microalloyed steels contain small amounts (< 0.1 wt%) of niobium, titanium, and/or vanadium [1] which lead to the formation of desirable carbonitride precipitates during thermo-mechanical controlled processing (TMCP). Mechanical-technological properties of microalloyed steels can be improved by optimization of TMCP parameters such as deformation degree during rolling, or cooling velocities.
In the past, TMCP optimization based on precipitate analysis relied on precipitate size distribution (PSD) and precipitate composition determination at different TMCP stages to monitor precipitation kinetics. Bulk [2,3,4,5,6,7,8,9,10] and replicated [11,12,13,14,15,16,17,18,19] samples have widely been investigated to characterize size, shape, and chemical composition of precipitates. The matrix dissolution technique (MDT), where steel samples are dissolved and the remaining precipitates characterized, is currently making its way from microalloyed steel research into industrial application [20, 21]. It provides data that is complementary to the conventional replica routes and can be combined with elemental analysis such as inductively coupled plasma – optical emission spectrometry (ICP-OES) to characterize the chemical composition of particle fractions.
Determining the precipitate number density in microalloyed steels is of great relevance to understand how it affects their mechanical-technological properties and how it can be modified through TMCP. Precipitate number densities cannot be determined via two-dimensional electron microscopy investigations on carbon extraction replicas and/or metallographically prepared cross sections of bulk samples. Further, the small volume fraction of precipitates (below 0.1%) limits the precipitate number density determination to high-resolution tomographic techniques such as X-ray diffraction in synchrotrons [22] or small-angle neutron scattering [5, 23].
Atom probe tomography (APT) has been used to estimate the number density of very small clusters or precipitates in steels at high number densities (> 1022 m−3) where the small investigated volumes of typically around 105 nm3 contain at least one precipitate [24, 25]. Xie et al. [26] employed APT to investigate age-hardening in a coiled Nb-microalloyed steel and could tie varying cluster size distributions and precipitate number densities to the respective bulk Vickers hardness. Webel et al. found that NbTi-microalloyed steel precipitates with greatly varying size distributions and number densities can appear simultaneously depending on the TMCP stage investigated [27]. Takahashi et al. investigated Cu precipitates in steel and found that an increase in mean precipitate size was accompanied by a decrease in precipitate number density [25]. The upper limit of APT for statistically robust precipitate number density determination is reached when the mean inter-precipitate distance is close to the dimensions of the APT specimen, typically a few tens to hundreds of nanometers. APT further was used for three-dimensional estimation of precipitate shape by careful inspection and calibration of the atom reconstruction [28, 29].
Precipitate number densities from MDT have not been reported in the literature to the best of our knowledge. MDT has been successfully applied to obtain distributions of precipitate size, shape, and chemical composition [20, 21], where it probes a much larger sample volume (several tens of mm3) than APT or replica techniques. Hegetschweiler et al. [30] report that an improved extraction protocol enables the analysis of sub-10 nm precipitates extracted from a NbTi-microalloyed steel.
Here, we combine MDT with AUC analysis of chemically extracted precipitates and APT to reconstruct their average number density in macroscopic sample volumes. Unlike APT the combination of MDT and AUC relies on indirect precipitate characterization. AUC is a fractionating characterization technique enabling analysis of colloidal systems [31,32,33]. Optical extinction of sedimenting precipitates is acquired to obtain a sedimentation coefficient distribution from which an extinction weighted PSD can be calculated. A Mie correction [34] is then applied to the extinction weighted PSD to determine a mass weighted PSD, prior to determination of a number weighted PSD. Number weighted PSDs are determined with Ångstrom resolution and high statistical significance in AUC [35].
In this contribution, number densities of NbC and NbTiCN precipitates in model Nb and NbTi-microalloyed steels were determined via APT and MDT. The discrepancies in precipitate number densities provided by the two techniques are discussed in view of the very different probed volumes. The validity of the standard assumptions on complete precipitation, sample homogeneity, and the resolving power of APT and AUC across different precipitate size ranges are discussed. We combined APT and MDT to cover a large precipitate number density range and to provide a holistic picture of the precipitate distribution in microalloyed steels.
Experimental procedures
Materials
Two high-Nb microalloyed low-carbon steels were obtained from Comtes FHT, Dobřany, Czech Republic, one containing titanium (“Steel NbTi” in the following), one without (“Steel Nb”). The elemental compositions as measured by the AG der Dillinger Hüttenwerke, Dillingen/Saar, Germany, with pulse discrimination analysis–optical emission spectrometry are given in Table 1. The concentration values are average values from measurements on three cross sections along the length of the cast blocks, each with nine measuring points (27 in total). The standard deviations across multiple measurements of the measured concentrations were below 0.002 wt% for most elements, indicating highly accurate measurements. Larger uncertainties occurred for a few samples and for some elements at higher concentrations such as Si, C, Mn, and Al; their standard deviations were around 0.005 wt%, but always below 0.01 wt%. Steel Nb was heat treated at AG der Dillinger Hüttenwerke (1000 °C for 1 h) to dissolve the cast structure and completely precipitate niobium. Steel Nb was then processed to ensure that all alloyed niobium forms fine NbC precipitates at a high number density. To this end, a 2 × 2 × 2 cm3 Nb steel volume was heated to 1250 °C for 30 min to ensure complete precipitate dissolution, quenched in water, heated to 690 °C, and kept there for 60 min to allow for complete precipitation of NbC, upon which the sample was again quenched in water. Thermal treatment was carried out in a furnace model HT 04/17 (Nabertherm, Lilienthal, Germany) in air. 5 mm of oxidized and decarburized material were mechanically removed from each side of the sample prior to chipping samples for characterization. The sample was selected because it is known to contain fine and homogeneous NbC.
Steel NbTi was slowly cooled after casting of a 500 kg ingot. During transformation from austenite to ferrite, fine interphase precipitation occurred because the solubility of Nb is much lower in ferrite than in austenite [36]. A fraction of Ti precipitated at higher temperatures to form larger cubic and insoluble TiN [37]. The sample was selected because it contains a broad size distribution of precipitates for the evaluation of the used methods and also a high precipitation density of fine NbTiCN.
Characterization methods
Atom probe tomography (APT)
APT was carried out in a LEAP 3000XHR (Cameca SAS, Gennevilliers, France). Specimens were cooled down to 60 K in an ultra-high vacuum (1.33 × 10–8 Pa). A direct current (DC) bias of up to 11 kV induced field-evaporation of single atoms toward a local electrode in front of a multi-plate detector. Upon reaching the bias threshold for field-evaporation, the voltage pulsed at 200 kHz with 15% of the DC bias to allow for controlled evaporation of ionized atoms. The DC bias was constantly adjusted to limit the detector signal to 3 ions per 1 000 pulses. The commercial software package IVAS 3.6.14 (Cameca SAS, Gennevilliers, France) was used for analysis of the detected ions. Atom positions in three-dimensional space were reconstructed based on crystallographic information that we applied to deconvolute the detector hit map [29]. Crystallographic poles were identified, and the map was fit with a model with tip radius and image compression factor as fitting parameters. The parameters were adjusted until the reconstructed Fe atoms had the known interplanar distance of iron for the respective crystallographic poles.
Apparent precipitate sizes ranged from 1 to 20 nm. Both iso-concentration surfaces (voxelization of 1 × 1 × 1 nm3 and a delocalization of 3 × 3 × 1.5 nm3 in x, y, and z direction, respectively) and a cluster-algorithm implemented in the software IVAS were applied to delineate precipitates. Reconstructed precipitates are likely magnified due to their comparably higher evaporation field strength [38]. We estimated the true diameters by the size of the void of reconstructed Fe atoms (indicated by low Fe atom density in the region of precipitates) rather than the precipitate elements themselves, following the approach of Breen et al. [28]. Nb iso-concentration values were chosen according to those Fe low-density regions. They ranged from 1 at% Nb for very small to around 3 at% for the largest Nb-precipitates. Alternatively, the cluster-detection algorithm implemented in IVAS was used [39]. Relatively high values were chosen for the maximum distance between two atoms belonging to the same cluster, Dmax, the envelope parameter L and the erosion distance parameter E. The values for Dmax and L were set to 2 nm and E to 1 nm. The minimum number of atoms defining a cluster (Nmin) was set to 4 atoms. Four Nb atoms needed to be within Dmax of each other to be attributed to the cluster to exclude random fluctuations of Nb atoms. The results were used to estimate a range for the true precipitate number densities.
Analytical ultracentrifugation (AUC)
A multiwavelength analytical ultracentrifuge was built using the design of the open AUC project on the basis of a preparative ultracentrifuge type Optima XL-80 K (Beckman Coulter, Brea, USA) [40]. Measurement cells with 12 mm titanium centerpieces were employed. Speed ramp AUC (SR-AUC) data were acquired between 1 and 50 kRPM (30 min at maximum speed) at 20 °C with a radial position of 6.7 cm at a wavelength of 450 nm. The measured data were evaluated using the freely available software HDR-Multifit [41, 42] before applying a Mie correction [43, 44] and determining a number weighted PSD with the free scientific python development environment Spyder (version 3.2.6) included with Anaconda. The code for Mie correction and PSD determination was introduced in previous work by Hegetschweiler [45]. The refractive index of NbC was set to 2.07 + 2.25i, that of NbTiCN to 1.72 + 1.55i. The NbC density was 7820 kg.m−3, that of NbTiCN to 6655 kg.m−3. The Disperbyk-2012 density was set to 1056 kg.m−3 and the ligand shell thickness to 5.7 nm [45].
Inductively coupled plasma–optical emission spectrometry (ICP-OES)
ICP-OES analysis of steel chippings was carried out with a Jobin Yvon Ultima 2 instrument (Horiba, Kyoto, Japan) equipped with a PEEK MiraMist nebulizer (PerkinElmer Inc., Waltham, USA) at 3.00 bar and 0.71 L.min−1. Niobium was detected at an emission wavelength of 313.079 nm and titanium at 337.280 nm.
Transmission electron microscopy (TEM)
Precipitate analysis was carried out with a JEM-2100(HR) transmission electron microscope (JEOL, Akishima, Japan) equipped with a LaB6 electron source at 200 kV. Precipitates were imaged with an Orius Model 832 camera (Gatan Inc., Pleasanton, USA).
Sample preparation
APT specimen preparation
The bulk samples were analyzed by APT at random sample positions across the polished steel surface. The samples were ground with SiC grinding paper up to 1200 mesh grid, polished on cloth disks with diamond suspension using decreasing particle sizes (6,3, and 1 µm), and finally oxide polished with a 0.05 µm silica particle suspension. Specimens were cut in a focused ion beam equipped scanning electron microscope (FIB-SEM) model Helios Nanolab™ 600 (Thermo Fisher Inc., Waltham, USA, formerly FEI Company, Hillsboro, USA) following the standard FIB lift-out method [46]. The lift-out bar was transferred to a multi-tip array of pre-fabricated Si posts and fixated with electrodeposited Pt. FIB annular milling with 30 kV and a 5 kV step for final sharpening produced specimen tips with a tip radius below 50 nm. APT specimen tips were imaged in FIB before and after field evaporation to compare the dimensions of probed material volume with the reconstructed tip volume that was adjusted to the crystallographic information extracted from the detector hit map.
Precipitate extraction for AUC
All chemicals were used as purchased without any further purification. Ultrapure water (UPW) was produced in a Milli-Q water-purification system (Advantage A10 and Elix 20, Millipore Corporation, Burlington, USA). 500 mg of steel chippings were dissolved in a 25 mL etching solution composed of 0.5 mol.L−1 H2SO4 prepared from conc. H2SO4 (95–97%, p.a., Sigma-Aldrich, Germany) by dilution with UPW and 0.1 vol.% Disperbyk-2012 (BYK Additives & Instruments, Wesel, Germany) at 70 °C following the extraction protocol developed by Hegetschweiler et al. [30] The reaction mixture was stirred at 200 RPM until dissolution completion and sonicated for 15 min in an ultrasonic bath type Sonorex Super RK 514 BH (BANDELIN electronic GmbH & Co. KG, Berlin, Germany). Suspension purification was carried out with a preparative ultracentrifuge type Optima XE-90 (Beckman Coulter, Brea, USA) at 504 300 RCF at 20 °C for 90 min. The suspension was centrifuged eight times. Purification was carried out by supernatant removal, residue redispersion via sonication, and supernatant replacement with fresh UPW after each centrifugation run. Completion of the eighth centrifugation step, supernatant removal, and residue redispersion via sonication led to a precipitate suspension of approximately 5 mL.
Sample dissolution for ICP-OES
A mixture of 25 mL HCl (≥ 37%, p.a. puriss, Sigma-Aldrich, Germany), 8 mL HNO3 (≥ 65%, p.a. puriss, Sigma-Aldrich, Germany), and 17 mL UPW was prepared. 5 mL of this acid mixture was combined with 1 mL of HF (50%, p.a. plus, Riedel-de-Haën, Germany) and used to etch 22.14 mg of Steel Nb chippings or 21.28 mg of Steel NbTi chippings in Teflon microwave vessels. The reaction mixture was irradiated with microwaves using a 0–800 W ramp in 1 h and constant 800 W for 1 h 30 min for Steel Nb. Steel NbTi was exposed to 0–600 W ramp in 1 h and a constant 600 W for 1 h 30 min. After cooling, the solutions were transferred to 50 mL flasks and filled up to the calibration marks with UPW prior to analysis.
Carbon extraction replica preparation
Carbon extraction replicas were prepared from steels Nb and NbTi. Polished steel surfaces (prepared analogously to the APT surfaces) were etched with 3 vol% alcoholic nitric acid (65% Suprapur®, Merck, Darmstadt, Germany) for 30 s, and a layer of carbon was deposited (K950X Turbo Evaporator, Emitech, now Quorumtech, Laughton, UK). The film was incised with a scalpel to form squares with 3 mm length and the sample was immersed into the acid solution for up to 30 min. Delaminated C-films were captured by 200 mesh TEM-grade copper grids and rinsed in a mixture of 30:70 ethanol and water prior to air drying.
APT measurement error estimation and statistical uncertainty determination
Measurement errors in precipitate number densities from APT are mainly due to incorrectly identified precipitates. The value from iso-concentration surfaces provides a lower limit and cluster analysis an upper limit for the true number of precipitates inside the APT volume. Precipitates located at specimen-edges were counted as 0.5 particles following the planimetric procedure approach [47].
The statistical uncertainty in average particle density was determined assuming a Poisson distribution for the number of precipitates per specimen (Eq. 1). If \(\lambda \epsilon {\mathbb{R}}\)+ and \(X\) is a real random variable. \(X\) follows Poisson’s law of parameter \(\lambda\) and \(X\hookrightarrow P(\lambda )\) if:
We determined the 95% probability range for the number of particles per investigated steel volume and precipitate identification technique based on the average precipitates per specimen. This range was converted to a precipitate number density range using the average specimen volume.
MDT measurement error estimation
We considered the random errors of the precipitate diameters and Nb/Ti concentrations. AUC analysis provides precipitate diameters at Ångström resolution [48] with a diameter measurement error of approximately 1%. ICP-OES analysis provides the niobium content with a measurement error of 1.7% for Steel Nb and 2.5% for Steel NbTi and the titanium content with a measurement error of 3.5% for Steel NbTi. We obtained these measurement errors by comparing the ICP-OES results (with errors that are typically below 0.1%) to the values listed in Table 1. We estimate the compound measurement error in precipitate number density from MDT to be approximately 5% for Steel Nb and 6% for Steel NbTi.
Results
Precipitate characterization via APT
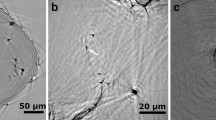
Reconstructed atom maps of Steel Nb and NbTi are displayed in Fig. 1a, b. From the detector hit maps of all APT experiments, the crystallographic information, as is exemplarily shown in Fig. 1c, was used to calibrate the atom reconstructions [29]. Approximately 50 and 100 particles were identified in total, respectively, and their chemical compositions were determined. We found that the Nb:C atomic ratios varied from 42:58 to 55:45 and concluded that precipitates in Steel Nb (Fig. 1a) contain Nb and C in equal atomic fractions to form NbC. Note that NbC1-x precipitates have been reported, too [49]. Precipitates in Steel NbTi (Fig. 1b) had the average composition Nb0.76Ti0.24C0.89N0.11 (NbxTi1-xCyN1-y). As with NbC in Steel Nb, metal and non-metal atoms were in near 1:1 atomic ratio. Nb precipitates were oblate, almost disk-shaped, whereas those in Steel NbTi were almost ideal spheres.
Reconstructed atom maps of several specimens of a Steel Nb showing Nb and C atoms and b Steel NbTi showing Nb, Ti, C, and N (from NbN peak) atoms. c Magnified rotated view from the reconstruction in the red frame in a) showing an oblate precipitate which lies on the (001) plane. The orientation was deduced from the crystal poles visible on the detector hit map (multiple detection events shown only). d Magnified view of red-framed proportion of reconstruction in b) with 1 at% iso-concentration surface of Nb overlaid
In Steel Nb, we mostly observed ellipsoid precipitates with a size of approximately 5 nm in the long axis and only 1–2 nm in the short axis. The crystal orientation from crystal pole analysis in APT indicated that the Nb-precipitates had their (100) habit planes oriented along the [100] or [010] direction (Fig. 1c). We also observed relatively larger precipitates reaching 15–20 nm in the long axis which were situated on C-decorated defects (lower right image of Fig. 1a). Precipitates in Steel NbTi, as exemplarily shown in Fig. 1b and d, have a near spherical shape in contrast to the oblate shape in Fig. 1a, c.
We calculated precipitate number densities by counting the precipitates in specimens of known volumes. The mean density in Steel Nb (Table 2) from iso-concentration surfaces (ICS) was 137.81 × 1021 m−3; cluster analysis (CA) indicated 193.56 × 1021 m−3. Assuming uniform densities throughout the sample, Student’s t analysis was used to estimate the errors of the means as 27.68 and 43.70 × 1021 m−3. Steel NbTi (Table 3) had mean densities of 104.90 × 1021 m−3 and 129.62 × 1021 m−3 for the two analysis methods with estimated errors of 39.52 and 57.40 × 1021 m−3. Cluster analysis consistently yielded higher values. The difference between the densities returned by both methods is larger for Steel Nb which had mostly smaller and plate like precipitates, whereas Steel NbTi had spherical particles which were more robustly detected with the iso-concentration method.
Precipitate number density determination via MDT
Chemically extracted precipitates were analyzed via AUC to determine an average precipitate mass (Eq. 2) and via ICP-OES to provide the total niobium and titanium masses.
with \({m}_{\mathrm{precipitate}}\) the average precipitate mass, \({\rho }_{\mathrm{core}}\) the density of NbC (7820 kg.m−3) or NbTiCN (6655 kg.m−3), and \(r\) the precipitate radius. The ligand shell contribution to the precipitate mass was neglected.
The calculation of densities (Eq. 3) requires the total carbon and/or nitrogen masses contained in precipitates.
with \({m}_{\mathrm{X}}\) the total mass of an element X contained in the precipitates, \({\rho }_{\mathrm{steel}}\) the steel density (7860 kg.m−3), and \({m}_{\mathrm{dissolved steel}}\) the mass of chemically dissolved steel.
For Steel Nb, we first assumed an Nb:C stoichiometry of 1:1 as reported in the literature [50] to determine the carbon mass and verified the assumption using our APT results. The carbon and nitrogen fractions for Steel NbTi were directly taken from the APT results. Alternatively, extraction replicas with AlxOy or Al films can be used to investigate the carbon to nitrogen ratio in precipitates. The suspension obtained by dissolving Steel Nb was analyzed via SR-AUC to determine a number weighted PSD. We found a monomodal size distribution (Fig. 2) with a modal precipitate diameter of 9.5 nm that corresponds to an average precipitate mass (Eq. 2) of 3.51 × 10–15 mg. The bulk Nb content of the steel from ICP-OES was 0.0821 wt% (Table 4), indicating a niobium mass of 0.42 mg in the steel chippings that we dissolved. The carbon mass of 0.05 mg was determined from the niobium mass by assuming a Nb:C stoichiometry of 1:1 [50] which is in accordance to APT results. A precipitate number density of 2.08 × 1021 m−3 with a measurement error of 0.10 × 1021 m−3 was determined for Steel Nb using Eq. 3.
The suspension obtained by dissolving Steel NbTi was analyzed via SR-AUC to determine a number weighted PSD. We found a monomodal precipitate population (Fig. 2) with a modal precipitate diameter of 10.2 nm that implied an average precipitate mass (Eq. 2) of 3.70 × 10–15 mg. The bulk Nb and Ti contents of the steel from ICP-OES were 0.0811 wt% and 0.0227 wt%, respectively (Table 4) equivalent to a niobium mass of 0.41 mg and a titanium mass of 0.11 mg contained in the steel chipping mass dissolved for AUC analysis. According to APT the carbon mass corresponds to 10% of the precipitate mass and the nitrogen mass to 1% of the precipitate mass. A precipitate number density of 2.48 × 1021 m−3 with a measurement error of 0.15 × 1021 m−3 was determined for Steel NbTi using Eq. 3.
Discussion
The precipitate number densities determined via APT and MDT for Steel Nb and NbTi (Table 5) were in the range expected from previously published findings [5, 24]. There was a large discrepancy between the values found by the different methods (Fig. 3). First, we discuss whether MDT analysis correctly assumes that all Nb (and Ti) precipitates, because solid solutions would falsify the results. Second, we discuss the sample homogeneity and possible effects of heterogeneity on the two methods.
Degree of Nb/Ti precipitation
The ICP-OES step after MDT does not differentiate between matrix and precipitate contributions. Residual dissolved Nb/Ti therefore directly translates into measurement errors and overestimated precipitate number densities. Metallurgical models let us estimate the magnitude of this error. Perrard et al. [23] established a time temperature transformation diagram for a very similar high-niobium alloy via small-angle neutron scattering. According to their data precipitation kinetics are above 90% completed. In the present work, the APT analysis of Steel Nb corroborated these results as less than 0.002 wt% Nb was measured inside the precipitate- or cluster-free matrix, equivalent to 97.5% precipitation of nominal Nb. Steel matrix of Steel NbTi also was practically depleted of all Nb (0.001 wt% Nb in solution) which is reasonable when considering the slow cooling of the ingot and the low solubility of Nb in ferrite.
Sample homogeneity
The microalloyed steel samples were chosen for homogeneity to enable a comparison of the 100 nm-scale APT with the mm-scale MDT analysis. Commercial steel samples are homogenous at the mm scale, with the possible exception of steels with center-line segregation precipitation. Analysis on the 100 nm scale, in contrast, is sensitive to the sampling position with respect to grain boundaries. We must consider whether the Nb distribution at the 100 nm scale found with APT can be compared to the millimeter-scale results from MDT.
Micro-segregation during melt solidification is known to change the Nb solute concentrations between dendritic and inter-dendritic regions by a factor of up to 1.6 at a length scale of hundreds of microns [51]. The homogenization treatment at 1250 °C of Steel Nb probably removed such heterogeneities. Steel NbTi did not undergo such a treatment, but APT results from Steel NbTi indicated less heterogeneity than Steel Nb and indicated that other alloying elements that do not precipitate (e.g., Si, Cu, and Ni) were evenly distributed. Nb has a comparatively large diffusivity and should be equally homogenous in spatial distribution. The standard error of APT precipitate density is therefore not influenced by chemical homogeneity of the sample material.
Defect structures may affect precipitate distribution, too. Steel Nb was isothermally held after a martensitic transformation and had a fine lath structure that is visible in carbon extraction replica and has a high grain boundary density. The lower right APT reconstruction in Fig. 1a illustrates that precipitates in the vicinity of the C-decorated martensite laths are much larger [52]. This can be explained by faster pipe-diffusion along such defects that enhanced nucleation and growth [53]. Steel NbTi had its microstructure transformed from austenite to ferrite during slow cooling and therefore fewer defects that interfere with the precipitation. This may contribute to the slightly higher uncertainties in the APT precipitate density in Steel Nb compared to Steel NbTi.
All APT measurements consistently indicated the presence of precipitates despite the small probed volume. We conclude that the samples of the steels studied here were sufficiently homogeneous for APT to give reasonable precipitate number density values. Even the lowest precipitate density found (8.90 × 1021 m−3, Table 3) in APT was 4 times above that from MDT, with an uncertainty that is mainly due to the small probed sample volume and small number of APT measurements.
Minimum Detectable Precipitate Sizes
The measurement errors of MDT depicted in Fig. 3 do not consider possible particle etching and loss [30] during chemical extraction that can bias the size distribution toward smaller diameters. Particles below a certain diameter can be etched during extraction and shrink, and smaller particles may be entirely consumed. Etching leads to an underestimated precipitate mass and an overestimated particle number density, complete dissolution of the smallest precipitates leads to the opposite. We compared APT and MDT results to find the minimum precipitate diameters that can be detected via AUC after MDT. Precipitate size distributions (PSDs) were calculated from the APT datasets using the method of Breen et al. [28] and converted to volume-equivalent sphere diameters that we use in the following. We found that AUC did not detect precipitates with diameters below 6 nm for Steel Nb and below 7 nm for Steel NbTi although APT indicated their presence (Fig. 4). The true precipitate diameter might be smaller than reconstructed by APT. Apparent precipitate size and shapes can be affected by the so-called local magnification that is caused by different evaporation field strengths of adjacent phases [38]. The Nb precipitates cause a higher field strength than iron [54] and their shape will be somewhat distorted, but the large difference in precipitate morphology between both steels indicates that rather the particle nucleation and growth is the reason for the morphology differences [26]. The high solution temperature used for Steel Nb, quenching and successive aging at 690 °C caused Nb precipitation from a highly supersaturated ferrite matrix that has a low solubility for Nb. The driving force for nucleation is large and results in finely dispersed precipitates; the mismatch of ferrite and NbC lattices favors the formation of precipitates over the formation path of Guinier–Preston zones [28, 55]. We additionally performed a TEM analysis of carbon extraction replicas (CERs) of both steels and compared the results to our MDT-AUC data. There was a clear offset between PSDs from TEM and AUC that we attribute to complete dissolution of the smallest precipitates and etching of all precipitates. The oblate precipitate shape in Steel Nb (Fig. 1) may aggravate etching. We conclude that MDT underestimates the true precipitate number density because of particle loss and estimated the resulting error in the particle number density by using modal diameters from CERs, which indicate particle densities of 14.26 × 1021 m−3 for Steel Nb and of 12.20 × 1021 m−3 for Steel NbTi, one order of magnitude above AUC-based data. A concurrent precipitate diameter reduction of approximately 5% [30] through undesired etching leads to a slightly underestimated precipitate mass and overestimated precipitate number density. Adding 5% to all precipitate diameters would imply densities of 1.80 × 1021 m−3 for Steel Nb and of 2.15 × 1021 m−3 for Steel NbTi. This implies that particle loss biases the precipitate number density from MDT more strongly than particle etching for the investigated microalloyed steels.
The minimum particle size detectable by APT is set by the detection efficiency (37% in the used machine). Clusters consisting of only a few atoms cannot be distinguished from solutes. The maximum particle size detectable by APT is set by the reconstructed volume. Particles which were not completely contained in the specimen were accounted for as half a particle. Particle densities from the iso-concentration method were consistently below that from cluster analysis. The cluster algorithm indicated a practically Nb-free steel matrix (0.001 to 0.002 at%), which implies that all Nb was detected as precipitate components and yielded an upper limit for the precipitate density. Some of the detected “clusters” may merely be groups of Nmin = 4 or more Nb atoms in solid solution that are close enough to appear as a cluster, which is possible due to the comparatively larger values for Dmax and L chosen in this study. The cluster algorithm thus probably overestimates the precipitate number density. The iso-concentration method is based on a (manual) visual inspection of images at different Nb iso-concentrations that are chosen such that all atoms that belong to precipitates are enveloped. A lower iso-concentration value reduces the fraction of atoms in solution that are mistaken for precipitated but increases the risk of missing precipitates. The lower limit for a feasible iso-concentration value depends on experimental factors including the local magnification caused by the difference in evaporation field strength required for carbonitrides and the Fe matrix [38]. The local magnification decreased the Nb concentration gradient at the precipitate-matrix interface. It is affected by particle geometry, too: The difference in precipitate number densities from cluster analysis and iso-concentration surfaces was larger for Steel Nb that contained oblate precipitates with a thickness down to a few atoms. Both evaporation field strength differences and oblate shapes lead the iso-concentration method to miss very small precipitates. The true precipitate number density therefore lies between the values determined via cluster analysis and iso-concentration surfaces.
Disregarding partial precipitates did not much change the results and led to densities of 117.29 to 170.47 × 1021 m−3 for Steel Nb and of 87.41 to 110.74 × 1021 m−3 for Steel NbTi. The result for Steel NbTi is on the same order as the values found by MDT after particle loss correction; the result for Steel Nb that contains smaller precipitates is one order of magnitude larger.
In summary, MDT provides a lower limit for the particle number density, while APT yields an upper limit. The main limitation of MDT is undesired particle loss, while APT is limited in the probed volume. Together, the methods provide a reliable range that is not available on other routes and consistent with metallurgical predictions.
Conclusions
We determined precipitate number densities of two microalloyed steels via APT and a novel technique based on MDT. The MDT precipitate number density determination technique relied on AUC analysis of chemically extracted precipitates and ICP-OES analysis of bulk steel chippings. We find that MDT provides a lower limit and APT an upper limit, because particle etching during chemical extraction affects the precipitate analysis in MDT and the small accessible volume limits APT. MDT and APT were found to be complementary in terms of accessible precipitate size ranges, chemical precipitate composition information, and precipitate morphology. Further reduction in undesired precipitate etching and loss, e.g., via electrolytic extraction, would benefit precipitate number density determination accuracy.
The MDT-APT combination can be used to analyze all TMCP steps. We are confident that the ability to determine precipitate number densities in all these process steps will lead to an improved understanding of the interrelation between processing parameters and mechanical-technological properties. Further development is required to cover TMCP steps with incomplete precipitation, where combinations of APT and MDT can potentially provide useful information. A holistic picture of the entire process will require the combination of APT and MDT with resistivity measurements and simulations.
Abbreviations
- APT:
-
Atom probe tomography
- AUC:
-
Analytical ultracentrifugation
- CA:
-
Cluster analysis
- CER:
-
Carbon extraction replica
- DC:
-
Direct current
- FIB:
-
Focused ion beam
- ICP-OES:
-
Inductively coupled plasma–optical emission spectrometry
- ICS:
-
Iso-concentration surfaces
- MDT:
-
Matrix dissolution technique
- PSD:
-
Precipitate size distribution
- SEM:
-
Scanning electron microscope
- SR-AUC:
-
Speed ramp analytical ultracentrifugation
- TEM:
-
Transmission electron microscopy
- TMCP:
-
Thermo-mechanical controlled processing
- UPW:
-
Ultrapure water
References
Gladman T (1997) The physical metallurgy of microalloyed steels. Inst Mater
Timokhina I, Miller MK, Wang J, Beladi H, Cizek P, Hodgson PD (2016) On the Ti-Mo-Fe-C atomic clustering during interphase precipitation in the Ti-Mo steel studied by advanced microscopic techniques. Mater Des 111:222–229. https://doi.org/10.1016/j.matdes.2016.08.086
Misra RDK, Jia Z, O’Malley R, Jansto SJ (2011) Precipitation behavior during thin slab thermomechanical processing and isothermal aging of copper-bearing niobium-microalloyed high strength structural steels: the effect on mechanical properties. Mater Sci Eng A 528:8772–8780. https://doi.org/10.1016/j.msea.2011.08.047
Kapoor M, O’Malley R, Thompson GB (2016) Atom probe tomography study of multi-microalloyed carbide and carbo-nitride precipitates and the precipitation sequence in Nb-Ti HSLA steels. Metall Mater Trans A 47:1984–1995. https://doi.org/10.1007/s11661-016-3398-6
Dhara S, Marceau R, Wood K, Dorin T, Timokhina I, Hodgson P (2018) Precipitation and clustering in a Ti-Mo steel investigated using atom probe tomography and small-angle neutron scattering. Mater Sci Eng A 718:74–86. https://doi.org/10.1016/j.msea.2018.01.070
Wang H, Somsen C, Li Y, Fries S, Detemple E, Eggeler G (2019) Effect of Nb on improving the impact toughness of Mo-containing low-alloyed steels. J Mater Sci 54:7307–7321. https://doi.org/10.1007/s10853-019-03374-2
Pereloma EV, Kostryzhev AG, Alshahrani A, Zhu C, Cairney JM (2014) Effect of austenite deformation temperature on Nb clustering and precipitation in microalloyed steel. Scr Mater 75:74–77. https://doi.org/10.1016/j.scriptamat.2013.11.026
Shen YF, Wang CM, Sun X (2011) A micro-alloyed ferritic steel strengthened by nanoscale precipitates. Mater Sci Eng A 528:8150–8156. https://doi.org/10.1016/j.msea.2011.07.065
Dong H, Chen H, Wang W, Zhang Y, Miyamoto G, Furuhara T, Zhang C, Yang Z, van der Zwaag S (2018) Analysis of the interaction between moving α/γ interfaces and interphase precipitated carbides during cyclic phase transformations in a Nb-containing Fe-C-Mn alloy. Acta Mater 158:167–179. https://doi.org/10.1016/j.actamat.2018.07.052
Takahashi J, Kawakami K, Tarui T (2012) Direct observation of hydrogen-trapping sites in vanadium carbide precipitation steel by atom probe tomography. Scr Mater 67:213–216. https://doi.org/10.1016/j.scriptamat.2012.04.022
Smith E, Nutting J (1956) Direct carbon replicas from metal surfaces. Br J Appl Phys 7:214–217. https://doi.org/10.1088/0508-3443/7/6/304
Craven AJ, He K, Garvie LAJ, Baker TN (2000) Complex heterogeneous precipitations in titanium-niobium microalloyed Al-killed HSLA steels – I. (Ti, Nb)(C, N) particles. Acta Mater 48:3857–3868. https://doi.org/10.1016/S1359-6454(00)00194-4
Craven AJ, He K, Garvie LAJ, Baker TN (2000) Complex heterogeneous precipitations in titanium-niobium microalloyed Al-killed HSLA steels – II. Non-titanium based particles. Acta Materialia 48:3869–3878. https://doi.org/10.1016/S1359-6454(00)00193-2
Béreš M, Weirich T, Hulka K, Mayer J (2004) TEM investigations of fine niobium precipitates in HSLA steel. Steel Res Int 75:753–758. https://doi.org/10.1002/srin.200405838
Scott C, Chaleix D, Barges P, Rebischung V (2002) Quantitative analysis of complex carbo-nitride precipitates in steels. Scr Mater 47:845–849. https://doi.org/10.1016/S1359-6462(02)00219-1
Charleux M, Poole WJ, Militzer M, Deschamps A (2001) Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Metall Mater Trans A 32:1635–1647. https://doi.org/10.1007/s11661-001-0142-6
Gong P, Palmiere EJ, Rainforth WM (2015) Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater 97:392–403. https://doi.org/10.1016/j.actamat.2015.06.057
Hansen SS, Vander Sande JB, Cohen M (1980) Niobium carbonitride precipitation and austenite recrystallisation in hot-rolled microalloyed steels. Metall Mater Trans A 11:387–402. https://doi.org/10.1007/BF02654563
Pavlina EJ, Van Tyne CJ, Speer JG (2015) Effects of combined silicon and molybdenum alloying on the size and evolution of microalloy precipitates in HSLA steels containing niobium and titanium. Mater Charact 102:35–46. https://doi.org/10.1016/j.matchar.2015.02.013
Hegetschweiler A, Borovinskaya O, Staudt T, Kraus T (2019) Single-particle mass spectrometry of titanium and niobium carbonitride precipitates in steels. Anal Chem 91:943–950. https://doi.org/10.1021/acs.analchem.8b04012
Lu J, Wiskel JB, Omotoso O, Henein H, Ivey DG (2011) Matrix dissolution techniques applied to extract and quantify precipitates from a microalloyed steel. Metall Mater Trans A 42:1767–1784. https://doi.org/10.1007/s11661-010-0579-6
Klinkenberg C, Klein H (2016) Synchrotron investigation on the precipitation behaviour of niobium microalloyed steel. Mater Sci Forum 879:948–953. https://doi.org/10.4028/www.scientific.net/msf.879.948
Perrard F, Deschamps A, Bley F, Donnadieu P, Maugis P (2006) A small-angle neutron scattering study of fine-scale NbC precipitation kinetics in the α-Fe-Nb-C system. J Appl Crystallogr 39:473–482. https://doi.org/10.1107/S002188980601301X
Shrestha SL, Xie KY, Zhu C, Ringer SP, Killmore C, Carpenter K, Kaul H, Williams JG, Cairney JM (2013) Cluster strengthening of Nb-microalloyed ultra-thin cast strip steels produced by the CASTRIP® process. Mater Sci Eng A 568:88–95. https://doi.org/10.1016/j.msea.2013.01.021
Takahashi J, Kawakami K, Kobayashi Y (2012) Consideration of particle-strengthening mechanism of copper-precipitation-strengthened steels by atom probe tomography analysis. Mater Sci Eng A 535:144–152. https://doi.org/10.1016/j.msea.2011.12.056
Xie KY, Zheng T, Cairney JM, Kaul H, Williams JG, Barbaro FJ, Killmore CR, Ringer SP (2012) Strengthening from Nb-rich clusters in a Nb-microalloyed steel. Scr Mater 66:710–713. https://doi.org/10.1016/j.scriptamat.2012.01.029
Webel J, Herges A, Britz D, Detemple E, Flaxa V, Mohrbacher H, Mücklich F (2020) Tracing microalloy precipitation in Nb-Ti HSLA steel during austenite conditioning. Metals 10:243. https://doi.org/10.3390/met10020243
Breen AJ, Xie KY, Moody MP, Gault B, Yen H-W, Wong CC, Cairney JM, Ringer SP (2014) Resolving the morphology of niobium carbonitride nano-precipitates in steel using atom probe tomography. Microsc Microanal 20:1100–1110. https://doi.org/10.1017/S1431927614012872
Gault B, Moody MP, Cairney JM, Ringer SP (2012) Atom probe crystallography. Mater Today 15:378–386. https://doi.org/10.1016/S1369-7021(12)70164-5
Hegetschweiler A, Staudt T, Kraus T (2019) An improved method for the matrix dissolution extraction of nanoparticles from microalloyed steel. J Mater Sci 54:5813–5824. https://doi.org/10.1007/s10853-018-03263-0
Lees EE, Gunzburg MJ, Nguyen T-L, Howlett GJ, Rothacker J, Nice EC, Clayton AHA, Mulvaney P (2008) Experimental determination of quantum dot size distributions, ligand packing densities, and bioconjugation using analytical ultracentrifugation. Nano Lett 8:2883–2890. https://doi.org/10.1021/nl801629f
Schindler T, Walter J, Peukert W, Segets D, Unruh T (2015) In situ study on the evolution of multimodal particle size distributions of ZnO quantum dots: some general rules for the occurrence of multimodalities. J Phys Chem B 119:15370–15380. https://doi.org/10.1021/acs.jpcb.5b08005
Schneider CM, Cölfen H (2019) High-resolution analysis of small silver clusters by analytical ultracentrifugation. J Phys Chem Lett 10:6558–6564. https://doi.org/10.1021/acs.jpclett.9b02755
Lechner MD (2005) Influence of Mie scattering on nanoparticles with different particle sizes and shapes: photometry and analytical ultracentrifugation with absorption optics. J Serb Chem Soc 70:361–369. https://doi.org/10.2298/JSC0503361L
Planken KL, Cölfen H (2010) Analytical ultracentrifugation of colloids. Nanoscale 2:1849–1869. https://doi.org/10.1039/C0NR00215A
Okamoto R, Borgenstam A, Ågren J (2010) Interphase precipitation in niobium-microalloyed steels. Acta Mater 58:4783–4790. https://doi.org/10.1016/j.actamat.2010.05.014
Kunze J, Mickel C, Backmann G, Beyer B, Reibold M, Klinkenberg C (1997) Precipitation of titanium nitride in low-alloyed steel during cooling and deformation. Steel Res Int 68:441–449. https://doi.org/10.1002/srin.199700580
Vurpillot F, Bostel A, Blavette D (2000) Trajectory overlaps and local magnification in three-dimensional atom probe. Appl Phys Lett 76:3127. https://doi.org/10.1063/1.126545
Larson DJ, Prosa TJ, Ulfig RM, Geiser BP, Kelly TF (2013) Local electrode atom probe tomography. Springer, New York. https://doi.org/10.1007/978-1-4614-8721-0
Cölfen H, Laue TM, Wohlleben W, Schilling K, Karabudak E, Langhorst BW, Brookes E, Dubbs B, Zollars D, Rocco M, Demeler B (2010) The open AUC project. Eur Biophys J 39:347–359. https://doi.org/10.1007/s00249-009-0438-9
Walter J, Peukert W (2016) Dynamic range multiwavelength particle characterization using analytical ultracentrifugation. Nanoscale 8:7484–7495. https://doi.org/10.1039/C5NR08547K
Walter J, Löhr K, Karabudak E, Reis W, Mikhael J, Peukert W, Wohlleben W, Cölfen H (2014) Multidimensional analysis of nanoparticles with highly disperse properties using multiwavelength analytical ultracentrifugation. ACS Nano 8:8871–8886. https://doi.org/10.1021/nn503205k
Mätzler C (2002) MATLAB functions for Mie scattering and absorption. Institute of Applied Physics, University of Bern. doi: https://doi.org/10.7892/boris.146550
Bohren CF (1983) Huffman, D.R. Absorption and scattering of light by small particles. Wiley. ISBN-13: 978–0–471–29340–8
Hegetschweiler A (2018) Colloidal analysis of particles extracted from microalloyed steels. PhD thesis, Saarland University, Germany, https://doi.org/10.22028/D291-27597
Thompson K, Lawrence D, Larson DJ, Olson JD, Kelly TF, Gorman B (2007) In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 107:131–139. https://doi.org/10.1016/j.ultramic.2006.06.008
ASTM E112–10 (2010) Standard test methods for determining average grain size, ASTM International, doi: https://doi.org/10.1520/E0112-10
Cölfen H, Pauck T (1997) Determination of particle size distributions with angström resolution. Colloid Polym Sci 275:175–180. https://doi.org/10.1007/s003960050068
Deardo AJ (2003) Niobium in modern steels. Int Mater Rev 48:371–402. https://doi.org/10.1179/095066003225008833
Tirumalasetty GK, Van Huis M, Fang C, Xu Q, Tichelaar F, Hanlon D, Sietsma J, Zandbergen H (2011) Characterization of NbC and (Nb, Ti)N nanoprecipitates in TRIP assisted multiphase steels. Acta Mater 59:7406–7415. https://doi.org/10.1016/j.actamat.2011.08.012
Roy S, Patra S, Neogy S, Laik A, Choudhary SK, Chakrabarti D (2012) Prediction of inhomogeneous distribution of microalloy precipitates in continuous-cast high-strength, low-alloy steel slab. Metall Mater Trans A 43:1845–1860. https://doi.org/10.1007/s11661-011-1045-9
Li YJ, Ponge D, Choi P, Raabe D (2015) Atomic scale investigation of non-equilibrium segregation of boron in a quenched Mo-free martensitic steel. Ultramicroscopy 159:240–247. https://doi.org/10.1016/j.ultramic.2015.03.009
Sellars CM (1998) Modelling strain induced precipitation of niobium carbonitride during hot rolling of microalloyed steel. Mater Sci Forum 284:73–82. https://doi.org/10.4028/www.scientific.net/MSF.284-286.73
Webel J, Weber L, Vardo E, Britz D, Kraus T, Mücklich F (2021) Particle encapsulation techniques for atom probe tomography of precipitates in microalloyed steels. Ultramicroscopy 223:113219. https://doi.org/10.1016/j.ultramic.2021.113219
Tingaud D, Maugis P (2010) First-principles study of the stability of NbC and NbN precipitates under coherency strains in α-iron. Comput Mater Sci 49:60–63. https://doi.org/10.1016/j.commatsci.2010.04.020
Acknowledgements
The authors would like to thank Andrea Jung for ICP-OES analysis and Dr. Johannes Walter for guidance in SR-AUC implementation.
Funding
Open Access funding enabled and organized by Projekt DEAL. L.W. and T.K. express their gratitude to the Aktien-Gesellschaft der Dillinger Hüttenwerke, Dillingen/Saar, Germany, for providing the financial funding of this work. J.W. and F.M. express their gratitude to Companhia Brasileira de Metalurgia e Mineracao (CBMM) for providing the financial funding of this work. Furthermore, J.W. and F.M. would like to thank the Saarland State Chancellery for financial support within the ZuMat Project funded by the European Regional Development Fund (ERDF). The Atom Probe was financed by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and the Federal State Government of Saarland (INST 256/298–1 FUGG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Catalin Croitoru.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weber, L., Webel, J., Mücklich, F. et al. Precipitate number density determination in microalloyed steels by complementary atom probe tomography and matrix dissolution. J Mater Sci 57, 12585–12599 (2022). https://doi.org/10.1007/s10853-022-07398-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07398-z