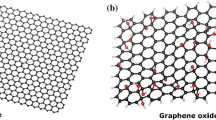
Abstract
Oxidation of graphite is the first important step for the industrial scale preparation of graphene by top-down method using graphite as a starting material. In this study, graphite was oxidized by economically viable and environmentally friendly thermal oxidation method. Pristine graphite (PG) was heated in a furnace under atmospheric condition between 200 and 1000 °C for specific time (5–20 min) and the samples were characterized through thermogravimetry analysis, X-ray photoelectron spectroscopy, Raman spectroscopy, X-ray diffraction, atomic force microscopy (AFM) and finally crystal simulation and molecular dynamic (MD) simulation were performed. The results exhibited that significant oxidation of PG took place when it was heated between 200 and 600 °C for shorter time (≤ 5 min), but partial reduction in oxidized graphite happened when heated for longer time (≥ 10 min) at same temperature. However, reduction of PG took place when it was heated between 800 and 1000 °C. The extent of oxidation was the maximum (17%) when graphite was heated at 400 °C for 5 min. Van der walls energy for graphite and oxygen system determined from MD simulation also found to be the maximum at 400 °C causing higher degree of oxidation. Finally, plausible mechanism of oxidation and reduction of graphite at different temperature and time was discussed based on experimental and simulation results.




















Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang DA, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Aristov VY, Urbanik G, Kummer K et al (2010) Graphene synthesis on cubic SiC/Si wafers: perspectives for mass production of graphene-based electronic devices. Nano Lett 10(3):992–995
Michon A, Vézian S, Ouerghi A, Zielinski M, Chassagne T, Portail M (2010) Direct growth of few-layer graphene on 6H-SiC and 3C-SiC/Si via propane chemical vapor deposition. Appl Phys Lett 97(17):171909
Yuan B, Bao C, Song L, Hong N, Liew KM, Hu Y (2014) Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem Eng J 237:411–420
Suk JW, Piner RD, An J, Ruoff RS (2010) Mechanical properties of monolayer graphene oxide. ACS Nano 4(11):6557–6564
Li X, Lau SP, Tang L, Ji R, Yang P (2014) Sulphur doping: a facile approach to tune the electronic structure and optical properties of graphene quantum dots. Nanoscale 6(10):5323–5328
Antolini E (2012) Graphene as a new carbon support for low-temperature fuel cell catalysts. Appl Catal B: Environ 123:52–68
El-Kady MF, Kaner RB (2013) Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nature Commun 4:1475
Kong XT, Khan AA, Kidambi PR, Deng S, Yetisen AK, Dlubak B, Hiralal P, Montelongo Y, Bowen J, Xavier S, Jiang K (2015) Graphene-based ultrathin flat lenses. ACS Photonics 2(2):200–207
Kuilla T, Bhadra S, Yao D, Kim NH, Bose S, Lee JH (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35(11):1350–1375
Obraztsov AN (2009) Making graphene on a large scale. Nature Nanotech 4(4):212–213
Torres L, Armas LG, Seabra AC (2014) Optimization of micromechanical cleavage technique of natural graphite by chemical treatment. Graphene 3(1):1–5
Camara N, Rius G, Huntzinger JR, Tiberj A, Mestres N, Godignon P, Camassel J (2008) Selective epitaxial growth of graphene on SiC. Appl Phys Lett 93(12):123503
Yang W, Chen G, Shi Z, Liu CC, Zhang L, Xie G, Cheng M, Wang D, Yang R, Shi D, Watanabe K (2013) Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nature Mater 12(9):792–797
Shen Y, Jing T, Ren W, Zhang J, Jiang ZG, Yu ZZ, Dasari A (2012) Chemical and thermal reduction of graphene oxide and its electrically conductive polylactic acid nanocomposites. Compos Sci Tech 72(12):1430–1435
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nature Nanotech 4(4):217
Botas C, Álvarez P, Blanco C, Santamaría R, Granda M, Gutiérrez MD, Rodríguez-Reinoso F, Menéndez R (2013) Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation–reduction of graphite oxide. Carbon 52:476–485
Sundaram RS, Gómez-Navarro C, Balasubramanian K, Burghard M, Kern K (2008) Electrochemical modification of graphene. Adv Mater 20(16):3050–3053
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339
Chen J, Yao B, Li C, Shi G (2013) An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229
Shen J, Hu Y, Shi M, Lu X, Qin C, Li C, Ye M (2009) Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem Mater 21(15):3514–3520
Li B, Wu C, Xu J, Hu D, Zhang T, Fang X, Tong J (2020) One-pot redox synthesis of graphene from waste graphite of spent lithium ion batteries with peracetic acid assistance. Mater Chem Phys 241(1):122397
Liu DF, Liang JZ (2014) Preparation of expandable graphite by ozone oxidation method. Adv Mater Res 1051:121–124
Chen Z, Liu Y, Zhang Y, Shen F, Yang G, Wang L, Zhang X, He Y, Luo L, Deng S (2018) Layered graphite prepared by an ozone aeration treatment as an anode material for lithium ion batteries. Int J Electrochem Sci 13:7282–7290
Osváth Z, Darabont A, Nemes-Incze P, Horváth E, Horváth ZE, Biró LP (2007) Graphene layers from thermal oxidation of exfoliated graphite plates. Carbon 45(15):3022–3026
Babout L, Marsden BJ, Mummery PM, Marrow TJ (2008) Three-dimensional characterization and thermal property modelling of thermally oxidized nuclear graphite. Acta Mater 56(16):4242–4254
Gao X, Zhan C, Yu X, Liang Q, Lv R, Gai G, Shen W, Kang F, Huang Z-H (2017) A high performance lithium-ion capacitor with both electrodes prepared from Sri Lanka graphite ore. Materials 10:414
Balasooriya NWB, Touzain Ph, Bandaranayake PWSK (2006) Lithium electrochemical intercalation into mechanically and chemically treated Sri Lanka natural graphite. J Phys Chem Solids 67(5–6):1213–1217
Kwan YCG, Ng GM, Huan CHA (2015) Identification of functional groups and determination of carboxyl formation temperature in graphene oxide using the XPS O 1s spectrum. Thin Solid Films 590:40–48
Yang D, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD, Stankovich S, Jung I, Field DA, Ventrice CA Jr, Ruoff RS (2009) Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47(1):145–152
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectrosc Relat Phenom 195:145–154
Saha T, Bhowmick AK, Oda T, Miyauchi T, Fujii N (2018) Influence of dianiline cross-linking systems on the structure, curing mechanism, and properties of polyacrylicester elastomer. Rubber Chem Technol 91(4):729–750
Zielke U, Hüttinger KJ, Hoffman WP (1996) Surface-oxidized carbon fibers: I surface structure and chemistry. Carbon 34(8):983–998
Casiraghi C, Ferrari AC, Robertson J (2005) Raman spectroscopy of hydrogenated amorphous carbons. Phy Rev B 72(8):085401
Tuinstra F, Koenig JL (1970) Raman spectrum of graphite. J Chem Phys 53(3):1126–1130
Saleem H, Haneef M, Abbasi HY (2018) Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater Chem Phys 204:1–7
Paulchamy B, Arthi G, Lignesh BD (2015) A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol 6(1):1–4
Li ZQ, Lu CJ, Xia ZP, Zhou Y, Luo Z (2007) X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45:1686–1695
Inoue M, Hirasawa I (2013) The relationship between crystal morphology and XRD peak intensity on CaSO4·2H2O. J Cryst Growth 380:169–175
Heerden JL, Swanepoel R (1997) XRD analysis of ZnO thin films prepared by spray pyrolysis. Thin Solid Films 299:72–77
Roisnel J, Rodrıguez-Carvajal J (2000) Win-PLOTR; Laboratoire Léon Brillouin (CEA-CNRS) centre d’Etudes de saclay: gif sur yvette cedex, France
Boultif A, Louër D (2004) Powder pattern indexing with the dichotomy method. J Appl Crystallogr 37(5):724–731
Louer D, Louer M (1972) Méthode d’essais et erreurs pour l’indexation automatique des diagrammes de poudre. J Appl Crystallogr 5(4):271–275
Boultif A, Louër D (1991) Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J Appl Crystallogr 24(6):987–993
Carvajal JR (2001) An introduction to the program FullProff 2000 (version July 2001). Laboratoire Léon Brillouin (CEA-CNRS) CEA/Saclay, 91191
Bhadra S, Kim NH, Lee JH (2010) Synthesis of water soluble sulfonated polyaniline and determination of crystal structure. J Appl Polym Sci 117(4):2025–2035
Bhadra S, Kim NH, Rhee KY, Lee JH (2009) Preparation of nanosize polyaniline by solid-state polymerization and determination of crystal structure. Polym Int 58(10):1173–1180
Fried JR, Li B (2001) Atomistic simulation of the glass transition of di-substituted polysilanes. Comput Theor Polym Sci 11(4):273–281
Pozuelo J, Baselga J (2002) Glass transition temperature of low molecular weight poly (3-aminopropyl methyl siloxane). A molecular dynamics study. Polymer 43(22):6049–6055
Isgro T, Phillips J, Sotomayor M, Villa E (2006) NAMD Tutorial Windows Version, 11–27
Martyna GJ, Tobias DJ, Klein ML (1994) Constant pressure molecular dynamics algorithms. J Chem Phys 101(5):4177–4189
Feller SE, Zhang Y, Pastor RW, Brooks BR (1995) Constant pressure molecular dynamics simulation: the Langevin piston method. J Chem Phys 103(11):4613–4621
Acknowledgement
This research is supported by CEAT Ltd., India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nair, S.S., Saha, T., Dey, P. et al. Thermal oxidation of graphite as the first step for graphene preparation: effect of heating temperature and time. J Mater Sci 56, 3675–3691 (2021). https://doi.org/10.1007/s10853-020-05481-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05481-x