Abstract
Covalent organic frameworks (COFs) are porous and crystalline materials which are formed based on the covalent interactions between the building monomers. These materials possess fascinating properties in terms of predesignable structure, controllable morphology, and manageable functionality which distinguished them from other polymers. COFs have also high chemical and physical stability, high surface area, and high adsorption capacity that these attributes make them excellent candidates for use in different fields. However, there are several approaches for the synthesis of COFs among which room-temperature synthesis approach is a green, versatile, and popular method which is due to its exceptional properties including simplicity, easy operation, and cost-effectiveness. In this regard, this review article presents a comprehensive view of the synthesis of COFs at room temperature as well as their applications, their limitations, and also their future perspectives.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Generally, the design and synthesis of new materials have always been welcomed by researchers. In the meantime, chemists have shown great interest in the synthesis of porous and crystalline structures using chemistry science [1, 2]. Porous and crystalline materials have ordered structure and also have separate molecular space which can interact with other molecules [3, 4]. In this regard, Yaghi et al. introduced porous and crystalline materials, namely covalent organic frameworks (COFs) for the first time in 2005, which were formed based on the condensation reaction between two-dimensional (2D) and three-dimensional (3D) organic building monomers [5, 6]. COFs contain elements like H, B, C, N, and O which are connected together by covalent interactions (Fig. 1) [3]. COFs have designable and controllable structures that introduce them as porous materials. Also, COFs have a lower density. In this regard, COFs keep their crystalline and ordered structure in different media [7, 8]. This high stability of COFs is related to the presence of strong covalent interactions. On the one hand, COFs are porous materials with the high surface area and subsequently high adsorption capacity. On the other hand, the presence of hydrogen bonding and π–π interactions in COFs improves the interactions and adsorption capacity of COFs. Hence, the flexible structure, high stability, and also high adsorption capacity make COFs as amazing platforms for use in different fields including catalysts [9, 10], semiconductors [11, 12], sensors [13, 14], pollutant treatment [15, 16], separation science [7, 17], and other applications [18, 19]. After the year 2007 that Yaghi and co-workers as pioneers in the synthesis of COFs introduced the first COFs, many efforts have been done for the preparation of different COFs and their applications. These studies have been applied to different synthesis approaches that have both advantages and disadvantages. Up to date, this is the first review for the investigation of room-temperature synthesis of COFs and their applications. So, in this review, we focused on the preparation of a comprehensive article for the synthesized COFs at room temperature, their application, their limitations, and also their future perspectives. Figure 2 shows the guidance image of this review article.
Structures of different COFs. Reproduced with permission [3].
COFs synthesis methods
The structure and morphology of the prepared COFs are particularly dependent on the kinds of applied building monomers and also the used synthesis approaches. The structure and morphology of prepared COFs can change by changing effective parameters on synthesis conditions like a solvent, temperature, catalyst, and so on. In this regard, different synthesis approaches have been applied for the fabrication of COFs. These methods are including solvothermal, ionothermal, microwave, sonochemical, mechanochemical, light-promoted, and room-temperature preparation methods. In the following, we will discuss each method separately.
Solvothermal method
In the solvothermal method, the COFs are prepared using temperature by mixing the building monomer in the autoclave [6]. In this method, the reaction conditions are highly dependent on the solubility and reactivity of building monomers and the reversibility of the reactions. Different parameters like time, temperature, solvent, and also the concentration of the applied catalyst are important in the morphology and structure of the prepared COF. It is worth mentioning that using the solvothermal method, some COFs can be synthesized on a large scale. The first 2D COFs were synthesized by the solvothermal method by Kaderi et al. [6]. In their study, COFs were synthesized at 120 °C for 72 h using dioxane and mesitylene as solvents. The pore sizes of the prepared COFs were in the range of 6–12 angstrom.
Ionothermal method
In an ionothermal method, ionic liquids or molten salts act as a solvent or catalyst. The reaction is performed at a high temperature. The most applied monomers in an ionothermal method are amorphous materials and lack long-range molecular orderings. The application of ionic liquids for the synthesis of COFs makes these methods simple, mild, and green approaches. The first COF was prepared using ionothermal method by Bojdys et al. [20].
Microwave method
In the microwave synthesis method, the synthesis of COFs is done using microwaves as an alternative energy source. Compared to solvothermal and ionothermal methods, microwave synthesis method needs lower reaction time and also forms the cleaner products. Also, in microwaves methods, we can follow the synthesis progress visually and can control the reaction temperature and pressure simultaneously. A property of the microwave solvent extraction method is that it could remove oligomers in the COFs more efficiently and the resulting COFs possess better porosity. Recently, Ji et al. [21] applied a rapid microwave synthesis approach for the fabrication of dioxin-linked COF for efficient microextraction of perfluorinated alkyl substances from water. The synthesis method is shown in Fig. 3. The prepared COF showed a high surface area (1254 m2 g−1). Also, the synthesized COF had high chemical stability in different solvents. The thermal stability of fabricated COF represented its good thermal stability up to 370 °C.
The preparation of COF by microwave method. Reproduced with permission [21].
Sonochemical method
The other COF synthesis approach is the sonochemical method. This method is fast and cost-effectiveness [22, 23]. Also, the effect of temperature and sonication waves as two main factors can investigate concurrently on the structure and morphology of the prepared COF. Most importantly, we can consider this method as a green method. The sonication waves not only can accelerate the desolvation of building monomer but also can introduce bubbles which can affect the prepared cavities in the structure of COF. Oliveira and co-workers [24] synthesized COF thin film using ultrasound irradiation. The speed of production of bubbles can affect the morphology of the prepared COF.
Mechanochemical method
One of the modern methods for the preparation of COF is a mechanochemical method. This method is simple, fast, solvent-free, and room temperature which is done only by grounding the pre-resources [25, 26]. The mechanochemical method is simple, economical, and environmental which can address the limitations of the other methods. In this regard, Biswal et al. [27] fabricated a COF by mechanochemical method (Fig. 4) [27]. SEM analysis proved that the spherical particles of prepared COF were covered with the agglomerate layer with relative sizes of 5 − 7 μm. The BET analysis showed that fabricated COF had high surface area of 537 m2 g−1 with a pore size distribution of 1.0 − 1.7 nm.
The preparation of COF by mechanochemical. Method. Reproduced with permission [27].
Light-promoted method
The other synthesis approach for COF is light-promoted method. In this method, COF is synthesized using simulated sunlight irradiation. Kim et al. [28] prepared a COF using simulated sunlight irradiation. The surface area of prepared COF was 598 m2 g−1 with a pore size distribution of 1.39 nm. Table 1 shows the comparison of different synthesis methods.
In spite of applicability and advantages, all these methods also have some disadvantages. Based on the applied method, the kind of prepared COF and also the structure and morphology of prepared COF are different. Some of these methods are faced with main drawbacks in terms of needing high temperature and most importantly physical and chemical stable building monomers at high temperature. Some of them also need expensive equipment for the preparation of COFs. These drawbacks have cheered researchers to use alternative methods for COFs fabrication.
Room temperature synthesis of COFs
One of the other main methods for the fabrication of COFs is the room-temperature method. Compared to other mentioned methods, the room-temperature method has exceptional properties in terms of simplicity and ease of operation which does not need temperature.
Most of the applied building monomers are not stable at high temperature that this issue enhances the importance of the application of room-temperature method for the preparation of COFs. In addition, the prepared COF at room temperature does not need gas protection step. Also, using temperature especially high-temperature synthesis approaches can be dangerous and hazardous since the nature of some of the used materials can change with changing temperature and produce side materials that can change the morphology of the prepared COF and subsequently decreases the stability of the prepared COF. In addition, the room-temperature synthesis approaches can be considered as green methods [29, 30].
Most of the synthesized COFs are time-consuming. Using the room-temperature method, the synthesis of COFs is more controllable. In spite of simplicity, the prepared COF at room-temperature methods shows high stability in harsh media and also has a high surface area and adsorption capacity.
Other advantages of the room-temperature synthesis approach are its applicability for applying on a large scale as a facile and effective synthetic method. The other significant point for using room-temperature synthesis method is that natural materials like biomolecules can also use as building blocks that are unstable in higher temperatures. (Based on the nature of the proposed biomolecule, the temperature is different.)
So, using room-temperature method can able us to apply natural materials like biomolecules which act as building blocks. One of the main problems in the synthesis of COFs is their fabrication using high-speed methods that not only introduce high crystallinity and porous structure but also have high stability. Although high-speed methods cannot introduce the mentioned properties, they are more suitable for large-scale production and also commercial and practical applications [31].
Up to now, between different applied room-temperature methods, steam-assisted conversion (SAC) introduces a high-speed and fast approach for the synthesis of different kinds of COFs with unique properties [32].
In this regard, a room-temperature COF was synthesized using a solution–suspension approach [33]. In this method, 1,3,5-triformylbenzene and p-phenylenediamine were dissolved in dioxane at room temperature. Then, acetic acid was added as a catalyst that changed the color of mixture to yellow. The mixture was kept for 3 days at room temperature. The achieved COF showed a good crystalline structure. The surface area of prepared COFs was achieved to be 410–1537 m2 g−1 with a pore size distribution of 2–50 nm. The main point about this study is the preparation of COFs using a continuous flow synthesis approach (Fig. 5). This method is not only fast and effective and can be used for large-scale and commercial production of COFs but also opened a new window for practical synthesis and application of COFs. This method requires stable building blocks with high solubility and also strong π interactions. This study was the first report for the synthesis of COFs by room-temperature continuous flow with a manufacturing speed of 41 mg h−1 at an enormously high space–time production of 703 kg m−3 day−1.
The preparation of COF by room temperature solution–suspension approach. Method. Reproduced with permission [33].
The fabricated COFs in this study displayed similar or even better properties in terms of crystallinity and porosity compared to COFs which were prepared by the solvothermal method. One of the main effective factors on the size and morphology of COFs is temperature. Temperature can change the structure and size of COFs from amorphous to crystalline. In this study, good solubility of monomers might be one of the driving forces for the formation of COFs under ambient conditions.
One of the other main factors on the formation of COFs is the conformation of applied building monomers which can change the size, morphology, and most importantly stability of prepared COF. In this study, with changing the building monomer with a different conformation, the COF was achieved which had lower BET surface area and poor stability. Different building monomers with a different conformation can change the possible interactions between monomers which can create steric hindrance between the building monomers and subsequently form COFs with different structures and stabilities.
In the other work, COF films were fabricated at room temperature through vapor-assisted conversion (VAC) method [32]. VAC is a variation of the well-known dry-gel conversion (DGC) and steam-assisted conversion (SAC) reported for the bulk and film syntheses of zeolites and other related compounds such as zeolitic imidazolate frameworks. VAC is based on the conversion of precursors in a cast solution layer into a continuous crystalline and porous film by exposure to a vapor of specific composition at moderate temperatures [34]. The prepared films had high quality with tunable thickness. These films exhibited mesoporous structure as well as textural porosity.
One of the excellent methods for the fabrication of COF films is the VAC method. The SEM images revealed a homogeneous surface consisting of small intergrown particles forming a continuous coverage on the substrate. The prepared films had a surface area of 990 m2 g−1.
One of the effective factors on the formation of COFs is the composition of the applied solvent. In this study, to investigate the effect of solvent vapor information on COF, different mixtures of mesitylene/dioxane were applied. Based on the results, the presence of mesitylene in the vapor mixture was crucial for the formation of a highly regular COF-5 structure. In this study, the role of time of formation and structure of COF was also evaluated. After 1 h of vapor exposure, a periodic phase starts to evolve and a gradual increase in periodicity is observed over 8 h.
Sun et al. [35] synthesized COF and then modified its pores for enhancing catalyst performance. In this study, the COF was synthesized using 1,3,5-tris(4-aminophenyl)-benzene (TPB) and 2,5-dimethoxyterephthalaldehyde (DMTA). In this method, the proposed polymer is placed in the channels which are related to the pores of COFs and enhance the catalytic activity of the proposed method. This approach benefits from the flexibility and enriched concentration of the functional moieties on the linear polymers, enabling the desired reaction environment in close proximity to the active sites, thereby impacting the reaction outcomes. The BET analysis represented the isotherm kind of IV with the surface area of 1510–1898 m2 g−1 for prepared COF.
In the other work, COF nanobars were fabricated in CO2/water solvent [36]. The presented method proved a facile, rapid, low-energy, and environmentally benign routes for the synthesis of COFs. This study was the first report for the fabrication of COFs in CO2/water as a solvent at room temperature. The most important things about the prepared COF were their crystalline structure, nanoscale size, and high surface area. Also, the COF has shown to be promising support for heterogeneous catalysts. Compressed CO2 is green, cheap, nonflammable, has adjustable properties, and most importantly can be easily recycled after use. CO2 can be dissolved in many solvents which can change the properties of these solvents, and subsequently, it can change the structure and morphology of the prepared COF. Also, CO2 and water can combine together and produce carbonic acid. In the following, carbonic acid can improve the speed of the proposed reaction.
Most importantly, to remove CO2, we can simply use the depressurization method. In addition, the produced CO2-dissolved water is also environmentally friendly. The prepared COF showed a good surface area (678 m2 g−1). It is main to say that the prepared COF at room temperature had a higher surface area (678 m2 g−1) compared to COF which was fabricated using a solvothermal method (410 m2 g−1). COF nanosheets as polymer nanoenhancer were synthesized by a novel and efficient strategy to exfoliation of COF [37].
The proposed COF was prepared at room temperature using a simple and fast method. This study applied a gram-scale method for preparing COFs nanosheets with butyl lithium as the intercalation agent which can address the limitation of the ball milling method. The TGA analysis showed that the prepared COF was stable up to 493 °C. In this study, the hydrophobic property of COF was investigated using the contact angle test. The prepared nanocomposite COF showed high hydrophobic property of prepared COF composite. To investigate the effect of different factors on the size of the prepared COF, a different amount of n-BuLi was used. Results showed that the size of prepared COFs nanosheets was not uniform, which may be caused by the exfoliation effect of n-BuLi.
Guan et al. [38] prepared a 3D COF using a fast, ambient temperature, and pressure ionothermal synthesis route. In this study, 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([BMIm][NTf2]) which is a liquid at room temperature (RT) was chosen as both solvent and catalyst for the Schiff base reaction. Also, the tetrahedral building block, tetrakis(4-formylphenyl)-methane (TFPM), reacted with the linear links of increasing size, p-phenylenediamine (PDA, 6.1 Å), 4,4′-diaminobiphenyl (DABP, 10.7 Å), or 4,4″-diamino-p-terphenyl (DATP, 15.3 Å) to produce the extended 3D interpenetrated. This study was a first report for using ionic liquid as a green solvent for the preparation of COF.
The reaction had high speed, and the applied ionic liquid can be used without losing its performance and activity. This COF was used for the separation of CO2/N2 and CO2/CH4. The prepared COF-3 showed a high surface area of 870 m2 g−1 with a pore volume of 0.56 mL g−1 and ideal adsorption selectivity of 24.4 for CO2/N2 and 21.5 for CO2/CH4. Based on the TGA analysis, the prepared COF contains 8 − 12% ionic liquids in their pores and shows high thermal stability (up to 450 °C) under nitrogen.
In the other study [39], COF thin film was fabricated at room temperature and applied as selective support for molecular separation (Fig. 6). In this study, a COF was fabricated using 1,3,5-triformylphloroglucinol (Tp) and diamine [2,2′-bipyridine-5,5′-diamine (Bpy)]. The bottom-up interfacial crystallization strategy was used to fabricate COFs powders as large-scale thin films under ambient conditions. The synthesis procedure is shown in Fig. 6. The prepared COF thin film showed a high surface area of 333–1151 m2 g−1 which makes them an amazing candidate for molecular separation. The prepared COF thin films were stable up to 400 ˚C. The SEM images proved the uniform thickness of prepared COF thin films. The TEM images proved the thin structure of prepared COF which was 3 mm [39]. Figure 6 shows the proposed mechanism for the COF thin-film formation and SEM and digital images of materials obtained at different stages [39].
Synthesis scheme of COF thin films. Reproduced with permission [39].
One of the popular approaches for fabrication of COFs is heterogeneous nucleation and growth. This method produces COFs with excellent crystallinity and high surface area. In the study which was done by Yuan and co-workers [40], the SiO2 nanoparticles were selected as the heterogeneous nuclei for COF growth which is due to uniform size and shape of SiO2 nanoparticles and their availability. Most importantly, after the fabrication of the COF layer, SiO2 nanoparticles can be etched to introduce a hollow structure. The other main point about this synthesis method is that it was done at room temperature, as shown in Fig. 7. The prepared COF showed high surface area of 1571.38 m2 g−1 with isotherm type I which proved the microporous structure of the prepared COF.
Proposed mechanism for the COF thin-film formation and SEM (a) and digital images (b) of materials obtained at different stages. Reproduced with permission [39].
Effective factors on size of COFs
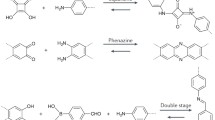
There are several factors that are effective in the size of COFs. One of the effective factors is the applied building blocks. The applied building blocks for the preparation of COFs have different sizes and different properties in terms of solubility and stability. In this regard, according to kind of the applied building blocks, the fabricated COFs have different sizes. There are different building blocks for the synthesis of COFs which include boroxine linkage, boronate ester, borosilicate, triazine, imine, hydrazine, borazine, squaraine, azine, phenazine, imide, double stage, spiroborate, C = C, amide, hypercoordinate silicon, urea, and 1,4-dioxin linkages. The other main factor on the synthesis of COFs is the time of reaction. The formation of COFs is based on the interaction of building blocks. To this end, changing reaction time can change the size and morphology of the prepared COF. The other main factor in the synthesis of COFs is applied solvents and catalysts. The change of solvent and catalyst can change the structure of prepared COF. The applied catalyst accelerates the formation of COFs which can directly change the structure of fabricated COF. Since the effect of these factors is very important, their optimization seems to be vital. In this regard, in most studies, the effect of these factors has been optimized. Temperature is another important factor for the synthesis of COFs which can change the size and morphology of COFs. Some of the COFs need temperature for synthesis.
Stability of COFs structure
Since the formation of COFs is based on the covalent interactions between building monomers, these materials have higher stability especially in 250 and 450 °C under an inert atmosphere. The stability of COFs is mostly related to the structures of knots, edges, and linkages. The strength of chemical bonds determines the overall chemical stability of COFs; in particular, the linkages are key to chemical stability. For example, boroxine- or boronate ester-linked COFs are not stable under humid or protic conditions. Unlike the boronate ester and boroxine-linked COFs, most nitrogen-based COFs, including imine, azine, hydrazone, squaraine, phenazine, imide, and triazine-linked COFs, exhibit remarkable hydrolytic stability. Compared with other nitrogen-based linkages, such as imine, azine, and hydrazine linkages, CS-COF and CTFs exhibit exceptional chemical stability owing to the formation of ring-fused phenazine and triazine units, respectively [41,42,43,44,45].
Application
One of the main area for application of COFs is their use in separation science for the determination of different compounds from the different matrix samples. This fact is due to the unique properties of COFs which makes them an amazing candidate for application as a sorbent in separation science. Many efforts have been done for fabrication and application of COFs in separation science.
In this regard, Liu et al. [46] synthesized COFs at room temperature and used it as a sorbent for solid-phase microextraction of phenols prior to gas chromatography-tandem mass spectrometry. In this study, the proposed COF was fabricated using Schiff base chemistry of 1,3,5-tri-(4-aminophenyl)benzene (TAPB) with 2,5-dimethoxyterephthalaldehyde (DMTA) in the presence of 3 M acetic acid and 1:1 mesitylene/dioxane. The solid-phase microextraction process is based on the evaporation of the target analyte and its adsorption on the surface of the sorbent. Hence, the applied sorbent not only must have a high surface area and also adsorption capacity but also must have high-temperature stability.
Hence, the strong adsorption affinity could be derived from hydrophobic and steric hindrance effects together with π − π affinity, H-bonding, van der Waals forces, and size-matching effect. Between different sorbents, COFs have these properties which distinguished them from other sorbents. The surface area of prepared COF was achieved to be 1560 m2 g−1. So, the high adsorption capacity is related to the presence of different interaction systems. Also, TGA analysis proved the stability of the proposed COF up to 470 °C. Based on the SEM images, the prepared COF showed spherical shape with size of 1–2 μm. The same sphere-like morphology and a dark area in sphere were also seen in TEM images. The proposed method showed high enrichment factors (1741–4265), low limits of detection (LOD) (0.0048–0.015 ng L−1), wide linearity (0.05–1,000 ng L−1), good precision (< 8.87%), and reproducibility (< 10.0%).
In the other work, COF and its bonded fiber were fabricated using in situ room-temperature synthesis approach and used for solid-phase microextraction of polychlorinated biphenyls in aquatic products [47]. The proposed COF was prepared using 1,3,5-tris-(4-formylphenyl)benzene (TFPB) and benzidine (BD). SEM images showed the presence of a uniform spherical COF coating on the surface of SS fibers, and the thickness of the coating is about 10 mm. The proposed sorbent had a satisfactory surface area (286 m2 g−1). The detection limits (LODs) (S/N = 3) and quantification limits (LOQs) (S/N = 10) of the TFPB-BD COF bonded SPME fiber for the polychlorinated biphenyls ranged from 0.07 mg L−1 to 0.35 mg L−1 and from 0.24 mg L−1 to 1.17 mg L−1, respectively.
In this study, the fabricated COF was compared with other fibers in terms of enhancement factor. Based on the results, the prepared COFs-based fiber in this study showed high enhancement factor. The larger enhancement factor of the TFPB-BD bonded fiber for PCBs resulted from hydrophobicity, π–π stacking interaction and steric hindrance effects between TFPB-BD and PCBs. These π–π and hydrophobic interactions between analytes and the aromatic frameworks of the TFPB-BD also make the TFPB-BD bonded fibers suitable for other aromatic toxic organic pollutants. Solid-phase extraction (SPE) is a popular method due to its unique properties.
Although SPE is simple and applicable, it is faced with main drawbacks including the getting stuck in pores of frits, introducing high pressure, and channeling the SPE column.[48, 49]. To address these problems, dispersive solid-phase extraction (DSPE) is an alternative method due to its excellent futures which not only can address the limitations of SPE mode but also improves the performance of the proposed method [50]. In addition, magnetic solid-phase extraction (MSPE) is a versatile method which not only has the properties of DSPE method but also reduces the extraction time and also enhances repeatability of the proposed method [51, 52].
These advantages have cheered researchers to fabricate and apply magnetic COFs for using them as sorbent in SPE method. In this regard, Chen et al. [53] synthesized magnetic COF nanobeads at room temperature using a facile synthesis approach and used it as a sorbent for MSPE of trace estrogens from human urine. In their study, 1,3,5-triformylbenzene (Tb) and benzidine (Bd) as two building blocks were applied. The prepared sorbent showed a good surface area of (202.18 m2 g−1) and high thermal stability. In spite of these advantages, the proposed method showed high precision and accuracy. This method was used for determination of organic targets in the urine samples.
In the other work, magnetic COF with 3D bouquet-like structure was synthesized using facile and room-temperature synthesis approach and used for enhanced extraction of organic targets [54]. In this study, a COF was fabricated from 1,3,5-triformylphloroglucinol (Tp) and p-phenylenediamine (Pa-1). Based on the TEM image, the COF layer was covered with Fe3O4 NPs which enhanced the stability of the Fe3O4 NPs. The magnetic COF exhibited isotherm type I which showed its microporous structure. The BET analysis showed satisfactory surface area of the prepared COF (247.8 m2 g−1) with the pore volume of 0.40 cm3 g−1 and size distribution of 0.4 − 2.0 nm. The proposed magnetic COF showed high thermal stability up to 300 °C.
Also, magnetic strength of prepared magnetic COF was 40.1 emu g−1 which enhanced the separation of COF from solution that not only reduces extraction time but also improves repeatability and recycling of the proposed method.
Gao and co-workers synthesized core–shell structured magnetic COF composite nanospheres for selective enrichment of peptides with simultaneous exclusion of proteins using simple, easy, fast, and room temperature synthesis approach [55]. In this study, monodisperse Fe3O4 nanoparticles were used as a core, and 1,3,5-triformylbenzene (TB) and benzidine (BD) were applied as two building blocks in the presence of dimethyl sulfoxide (DMSO). The most important things about this study are short synthesis time (5 min) and room-temperature synthesis method. The prepared COF had amazing properties in terms of high stability, high surface area, good magnetic strength, and so on. The acetic acid in this study acts as a catalyst which not only hydrolyzes amine groups but also accelerates the reaction of the condensation process between building monomers. Most importantly, acetic acid enhances the crystallinity of the prepared COF. SEM and TEM images of the prepared COF and magnetic COF represented the spherical structure of the prepared COF with a core–shell structure. The saturated magnetization value of the prepared Fe3O4@COF was 41.5 emu g−1 which improved the separation of sorbent from solution and reduced the extraction time.
In the following, core–shell magnetic COF was fabricated using a room-temperature method for efficient enrichment of peptides and simultaneous exclusion of proteins [56]. The proposed COF was synthesized using 1,3,5-tris (4-aminophenyl) benzene (TAPB) and terephthaldicarboxaldehyde (TPA) as organic ligands in dimethyl sulfoxide (DMSO). The TGA analysis proved the stability of prepared sorbent up to 400 °C. The COF showed high stability in acidic and basic media. The BET analysis of sorbent proved that it had the isotherm kind IV and surface area and average pore diameter of 178.87 m2 g−1 and 3.92 nm, respectively.
In the other study, bisphenols were extracted from human serum sample using core–shell structured magnetic COF nanocomposites based on MSPE [57]. In this study, a COF was fabricated using 1,3,5-tris(4-aminophenyl)benzene (TAPB) and terephthaldicarboxaldehyde (TPA) as two building units in the presence of dimethyl sulfoxide (DMSO). The as-prepared Fe3O4@COF nanocomposites with core–shell structure possessed high specific surface area (181.36 m2/g), uniform mesoporous size (~ 3.6 nm), high saturation magnetization (42.7 emu/g), and excellent thermal and chemical stability, rendering it as an ideal adsorbent with high adsorption efficiency and size selectivity. The prepared Fe3O4@COF showed exclusive properties. The effect of effective parameters on extraction recovery was also discussed. Also, the proposed method and sorbents proved high precision and accuracy.
One of the applications of COFs is their use as a membrane for the separation of different molecules like dyes and organic molecules.
In this regard, Wang et al. [58] prepared COFs using polymeric substrates for dye separation using a unidirectional diffusion synthesis approach. COFs were used as membrane due to their unique properties. The preparation process and using process are shown in Fig. 8 [58]. The applied membrane showed excellent selectivity and high applicability.
Schematic of preparation process of the COFs-based membranes via the UDS method. (a) Schematic illustration of diffusion cell for TpPa growth by UDS. (b) Schematic illustration of the formation TpPa selective layer on the top sides of PVDF support via unidirectional diffusion of Pa and PTSA (catalyst) molecules through the pores of the PVDF support to react with Tp molecules. c Synthesis of TpPa with a keto form through the condensation of Tp (blue) and Pa (red). d The schematic illustration of intergrowth appearance of the TpPa/PVDF membranes after synthesis. Reproduced with permission [58].
In this study, the p-phenylenediamine (Pa) aqueous solution and 1,3,5-triformylphloroglucinol (Tp) were used in n-hexane solution for fabrication of COF. The membrane fabricated with a synthesized duration of 24 h exhibits a superior selectivity for dyes (> 90%) and appreciable water permeance (60 L m−2 h−1 bar−1). The stability of the prepared membrane was evaluated at room temperature using a 12 h continuous separation test. Also, the stability of COF was investigated using strong acid (2 M HCl) and strong base (2 M NaOH) aqueous solution for a week and comparing with those of the membrane without treatment.
In other study, COF was fabricated using one-pot synthesis method at room temperature in water [59]. The applied method produced sub-20 nm crystalline imine-based COF particles. Most importantly, in this study, with combination of experimental and computational studies, the authors evaluated the mechanisms and forces underlying the formation of such imine-based COF colloids in water. Further, the authors showed that their method can be used to process the colloidal solution into 2D and 3D COF shapes as well as to generate a COF ink that can be directly printed onto surfaces. The applied method can open new vistas for application of this kind of COFs in different fields.
One of the main applications of room-temperature synthesized COFs is removal and treatment of pollutants like heavy metals. This fact is due to exclusive properties of COFs in terms of high surface area, high adsorption capacity, and high stability which make them as good candidate for application in different fields. In this regard, the COF was fabricated at room temperature [60]. Then, Ag nanoparticles were anchored in prepared COF and used for removal of mercury from acidic wastewater. The proposed COF was prepared using in situ and one-step solution infiltration approach. The most important thing about fabricated COF is its high stability in acidic media. More importantly, the Ag NPs@COF composite exhibited high removal rate (99%), ultrahigh Ag atom utilization (150%), high selectivity and stability, and reusability for Hg(II) removal from acidic aqueous solutions.
One of the interesting applications of room-temperature synthesized COF is batteries. To this end, Xu et al. fabricated multifunctional COF and used it in lithium metal batteries [61]. Due to the lithiophilicity of COF-LZU1 and the interaction with electrolyte anions, COF-LZU1 can immobilize anions and disperse Li ions. Therefore, COF-LZU1 as artificial protective layer promotes a dense and smooth Li deposition without dendrites, rendering an effective approach to enable the next-generation high energy/power Li metal batteries. The COF-LZU1 was prepared at room temperature. In this study, LZU1 (COF-LZU1) is introduced to serve as a protective layer in between the Li anode and separator. Because of the interaction between bis(trifluoromethanesulfonyl)imide (TFSI) anions and aldehyde functional groups in COF-LZU1, TFSI are immobilized on the COF-LZU1, alleviating the impact of space charge and hence suppressing the dendrite growth.
The other application of COFs is their use for detection and sensing of different analytes. One of the simple and reliable analytical methods for detection of different analytes is colorimetric. Due to large π-conjugated system of COFs, they can be used in colorimetric system for detecting different analytes. To this end, Ai and co-workers used COF for visual colorimetric detection of carcinogenic 3, 3′-diaminobenzidine [62].
Due to unique properties of COFs and especially room-temperature synthesized COFs, they can be useful for application in different fields including:
-
Separation science
-
Treatment of pollutants
-
Application as catalyst and photocatalyst
-
Gas adsorption
-
Use in the liquid phase
-
Medicine and drug delivery
-
Fluorescence
-
Sensors
-
Solar cells
Table 2 shows the differences of some COFs which fabricated at room temperature with some other hollow porous materials which are prepared at room temperature [33, 35, 36, 38,39,40, 46, 47, 53, 54, 56, 57, 63,64,65,66,67,68,69].
Limitations
Although the room-temperature synthesis method has amazing properties, it is faced with the main drawbacks. The building monomers play the most important role in the synthesis of COFs since they can control the structure and morphology of the prepared COFs. Using a room-temperature synthesis approach limits us to fabricate special kinds of COFs. This is due to the fact that most of the building monomers are not soluble at room temperature and they need temperature especially high temperature for preparation. In this regard, using a room-temperature method, it is impossible for us to fabricate different kinds of COFs. Using temperature, we can change the nature of building monomers and introduce new monomers. One of the main areas about application of COFs is their combination with other materials like nanoparticles, metal–organic frameworks (MOFs), and molecularly imprinted polymers (MIPs). The combination of COFs with other materials can improve their properties and also their application. But, most of nanoparticles, MOFs, and MIPs need temperature for synthesis. Indeed, temperature plays important role in synthesis of these materials. For example, the MIPs which have fabricated using radical polymerization need temperature for activation of the initiator. Or, some materials and reagents for synthesis of MOFs and MIPs need temperature to dissolve. These drawbacks limit the application of room-temperature synthesis of COFs.
Future and perspectives
One of the main aspects in synthesis methods is the synthesis and using of selective sorbent. Indeed, the applied sorbents must have not only stability, high surface area, and high adsorption capacity but also selectivity. This fact encouraged chemists and researchers to design and synthesis selective sorbent with high stability and high surface area. In this regard, most of the researches have been focused on synthesis of molecularly imprinted polymers (MIPs) as selective materials. MIPs are a man-made, selective, and versatile material with predefined recognition sites which are formed during the polymerization process [70, 71]. After removal of target analyte, the recognition sites can act as selective cavities based on the size, shape, and functional groups of the applied analyte [72, 73]. MIPs are not only selective but also are simple, easy to synthesize, and cheap [74, 75]. In spite of selectivity, MIPs are faced with main drawback, namely low adsorption capacity. To address this main limitation, many efforts have been performed. One of the interesting approaches is the combination of MIPs as selective materials with COFs as versatile materials with fascination properties in terms of high stability, high surface area, and also high adsorption capacity. Indeed, a combination of MIPs with COFs introduces novel kinds of sorbent that have both properties of MIPs and COFs. In spite of selectivity, most of MIPs and COFs have been prepared using toxic and hazardous materials and multi-step synthesis approaches. These approaches not only are hazardous but also are time-consuming and expensive. To remove these limitations, green synthesis approaches can be an ideal choice for the fabrication of green and room-temperature COFs. Up to know, most of the COFs have been synthesized using organic solvents which is not in the line of green chemistry. To remove this problem, synthesis of a new kind of building monomers that are soluble in water can be an ideal choice. This approach not only decreases using hazardous solvents but also enhances the water compatibility of the prepared COFs. Since one of the main areas for application of COFs is separation fields, this approach can enhance the performance of the prepared COFs for the determination of different compounds especially in water and aqueous media like water and biological fluids like urine, blood, and plasma samples. Most of the prepared COFs are not conductive which limits their application in the fields like water splitting, photodegradation, and also catalyst. To address these limitations, combination of COFs with conductive materials like MOFs and metal-oxide nanoparticles can open a new window for the widespread application of COFs. By the way, most of MOFs, MIPs, and also metal-oxide nanoparticles can be synthesized at room temperature which can improve their applicability. To enhance surface area and also adsorption capacity of the prepared COFs, we can use multi-functional building monomers which not only can improve the adsorption capacity but also can enhance the stability of the prepared COFs. Also, we can use natural building monomers which are stable at room temperature for synthesis of COFs.
Conclusion
In this review article, we focused on the synthesis of covalent organic frameworks as versatile materials with amazing properties in terms of porous and crystalline structure, tunable structure, high stability, high surface area, and also high adsorption capacity. Between different synthesis approaches that have been applied for the preparation of covalent organic frameworks, a room-temperature method is a versatile approach with unique properties in terms of easy, simple, fast, and green approaches. In this study, we focused on the properties of room-temperature synthesis approaches for preparation of covalent organic frameworks. Also, we compare this method with some other reported methods. Finally, the limitations and also future perspectives of the room-temperature synthesis approach were discussed. This study confirmed that room-temperature method can be used in different areas which is due to its fascinating properties. Although this method is faced with the main drawbacks, they can be solved and can be the subject of many types of research in the near future. Indeed, this review article will open a new window for the researchers who are willing to work and study on covalent organic frameworks.
References
Gardel ML (2013) Nature 493:618
Fukui T, Kawai S, Fujinuma S et al (2017) Nat chem 9:493
Sharma RK, Yadav P, Yadav M et al (2020) Mater Horiz 7:411
Kouwer PH, Koepf M, Le Sage VA et al (2013) Nature 493:651
Huo J, Luo B, Chen Y (2019) ACS Omega 4:22504. https://doi.org/10.1021/acsomega.9b03176
El-Kaderi HM, Hunt JR, Mendoza-Cortés JL et al (2007) Science 316:268
Wang Z, Zhang S, Chen Y, Zhang Z, Ma S (2020) Chem Soc Rev 49:708
Nguyen HL, Gropp C, Yaghi OM (2020) J Am Chem Soc 142:2771
Ding S-Y, Gao J, Wang Q et al (2011) J Am Chem Soc 133:19816
Díaz U, Corma A (2016) Coord Chem Rev 311:85
Dogru M, Bein T (2014) Chem Commun 50:5531
Babu HV, Bai MM, Rajeswara Rao M (2019) ACS Appl Mater 11:11029
Dalapati S, Jin S, Gao J, Xu Y, Nagai A, Jiang D (2013) J Am Chem Soc 135:17310
Yang L, Jin Y, Wang X et al (2020) Adv Electron Mater 6:1901169
Sun Q, Aguila B, Perman J et al (2017) J Am Chem Soc 139:2786
Huang N, Zhai L, Xu H, Jiang D (2017) J Am Chem Soc 139:2428
Zhao L, Lv W, Niu X, Pan C, Chen H, Chen X (2020) J Chromatogr A 1615:460722
Song Y, Sun Q, Aguila B, Ma S (2019) Adv Sci 6:1970011
Kang Z, Peng Y, Qian Y et al (2016) Chem Mater 28:1277
Bojdys MJ, Jeromenok J, Thomas A, Antonietti M (2010) Adv Mater 22:2202
Ji W, Guo Y-S, Xie H-M, Wang X, Jiang X, Guo D-S (2020) J Hazard Mater 397:122793. https://doi.org/10.1016/j.jhazmat.2020.122793
Bang JH, Suslick KS (2010) Adv Mater 22:1039
Cintas P, Luche J-L (1999) Green Chem 1:115
Oliveira CJF, Freitas SKS, de Sousa IGPP, Esteves PM, Simao RA (2020) Colloids Surf A 585:124086. https://doi.org/10.1016/j.colsurfa.2019.124086
Friščić T (2010) J Mater Chem 20:7599
James SL, Adams CJ, Bolm C et al (2012) Chem Soc Rev 41:413
Biswal BP, Chandra S, Kandambeth S, Lukose B, Heine T, Banerjee R (2013) J Am Chem Soc 135:5328. https://doi.org/10.1021/ja4017842
Kim S, Choi HC (2019) Commun Chem 2:1
Poole CF, Lenca N (2015) TrAC Trends Anal Chem 71:144. https://doi.org/10.1016/j.trac.2014.08.018
Brahmachari G (2015) ACS Sustain Chem Eng 3:2350. https://doi.org/10.1021/acssuschemeng.5b00826
Jiang Y, Huang W, Wang J et al (2014) J Mater Chem A 2:8201
Medina DD, Rotter JM, Hu Y et al (2015) J Am Chem Soc 137:1016
Peng Y, Wong WK, Hu Z et al (2016) Chem Mater 28:5095. https://doi.org/10.1021/acs.chemmater.6b01954
Virmani E, Rotter JM, Mähringer A et al (2018) J Am Chem Soc 140:4812. https://doi.org/10.1021/jacs.7b08174
Sun Q, Tang Y, Aguila B et al (2019) Angew Chem 131:8762
Zhang F, Zhang J, Zhang B et al (2018) Chemsuschem 11:3576
Mu X, Zhan J, Wang J et al (2019) J Colloid Interface Sci 539:609
Guan X, Ma Y, Li H et al (2018) J Am Chem Soc 140:4494
Dey K, Pal M, Rout KC et al (2017) J Am Chem Soc 139:13083
Yuan Y-C, Sun B, Cao A-M, Wang D, Wan L-J (2018) Chem Commun 54:5976
Sun T, Xie J, Guo W, Li DS, Zhang Q (2020) Adv Energy Mater 10:1904199
Du Y, Mao K, Kamakoti P et al (2013) J Mater Chem A 1:13171
Li H, Li H, Dai Q, Li H, Brédas JL (2018) Adv Theory Simul 1:1700015
Du Y, Mao K, Kamakoti P et al (2012) Chem Commun 48:4606
Du Y, Calabro D, Wooler B et al (2015) Chem Mater 27:1445
Liu L, Meng W-K, Li L et al (2019) Chem Eng J 369:920
Guo J-X, Qian H-L, Zhao X, Yang C, Yan X-P (2019) J Mater Chem A 7:13249
Bagheri AR, Ghaedi M (2020) Carbohydrate Polym 236:116102
Arabi M, Ghaedi M, Ostovan A (2017) ACS Sustain Chem Eng 5:3775
Bagheri AR, Arabi M, Ghaedi M et al (2019) Talanta 195:390
Gholami H, Arabi M, Ghaedi M, Ostovan A, Bagheri AR (2019) J Chromatogr A 1594:13
Ostovan A, Ghaedi M, Arabi M (2018) Talanta 179:760
Chen L, Zhang M, Fu F, Li J, Lin Z (2018) J Chromatogr A 1567:136
He S, Zeng T, Wang S, Niu H, Cai Y (2017) ACS Appl Mater Interfaces 9:2959
Gao C, Lin G, Lei Z, Zheng Q, Lin J, Lin Z (2017) J Mater Chem B 5:7496
Lin G, Gao C, Zheng Q et al (2017) Chem Commun 53:3649
Chen L, He Y, Lei Z et al (2018) Talanta 181:296
Wang R, Shi X, Zhang Z et al (2019) J Membr Sci 586:274
Franco C, Rodríguez-San-Miguel D, Sorrenti A et al (2020) J Am Chem Soc 142:3540. https://doi.org/10.1021/jacs.9b12389
Wang L, Xu H, Qiu Y et al (2020) J Hazard Mater 389:121824. https://doi.org/10.1016/j.jhazmat.2019.121824
Xu Y, Zhou Y, Li T et al (2020) Energy Storage Mater 25:334. https://doi.org/10.1016/j.ensm.2019.10.005
Ai R, He Y (2020) Sensors Actuators B: Chem 304:127372. https://doi.org/10.1016/j.snb.2019.127372
Song X, Yang P, Wu D et al (2020) Chem Phys 531:110655. https://doi.org/10.1016/j.chemphys.2019.110655
Oveisi M, Mahmoodi NM, Asli MA (2019) J Cleaner Prod 222:669. https://doi.org/10.1016/j.jclepro.2019.03.066
Chen Z, Wang X, Noh H et al (2019) CrystEngComm 21:2409. https://doi.org/10.1039/C9CE00213H
Yuan B, Wang X, Zhou X, Xiao J, Li Z (2019) Chem Eng J 355:679. https://doi.org/10.1016/j.cej.2018.08.201
Sadat SA, Ghaedi AM, Panahimehr M, Baneshi MM, Vafaei A, Ansarizadeh M (2019) Appl Surf Sci 467–468:1204. https://doi.org/10.1016/j.apsusc.2018.10.274
Huang L, Cai J, He M, Chen B, Hu B (2018) Ind Eng Chem Res 57:6201. https://doi.org/10.1021/acs.iecr.7b05294
Dong C, Li Z, Zhang L et al (2019) Diam Relat Mater 92:32. https://doi.org/10.1016/j.diamond.2018.12.011
Bagheri AR, Ghaedi M (2019) Int J Biol Macromol 139:40
Arabi M, Ostovan A, Bagheri AR et al (2020) Talanta. https://doi.org/10.1016/j.talanta.2020.120933
Gholami H, Ghaedi M, Arabi M, Ostovan A, Bagheri AR, Mohamedian H (2019) ACS Omega 4:3839
Gholami H, Ghaedi M, Ostovan A, Arabi M, Bagheri AR (2019) Microchim Acta 186:702
Guo X, Li J, Arabi M, Wang X, Wang Y, Chen L (2020) ACS Sens 5:601. https://doi.org/10.1021/acssensors.9b02039
Yang Q, Li C, Li J et al (2020) Nanoscale 12:6529
Acknowledgements
This work was financially supported by Graduate School and Research Council of Yasouj University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Chris Cornelius.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagheri, A.R., Aramesh, N. Towards the room-temperature synthesis of covalent organic frameworks: a mini-review. J Mater Sci 56, 1116–1132 (2021). https://doi.org/10.1007/s10853-020-05308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05308-9