Abstract
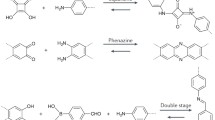
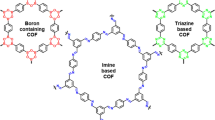
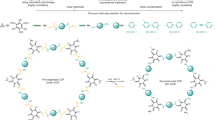
Covalent organic frameworks (COFs) are periodically well-organized polymeric skeletons of organic monomer units connected through strong covalent linkages to form stable crystalline materials. The specific bonding among the organic monomer unit constructs a regular and porous skeleton, and therefore, these COFs are considered as highly ordered and uniform materials. COFs have recently appeared as heterogeneous catalysts for various organic transformations and photocatalytic reactions. These properties are arisen due to the reticular design of these materials, and this reticular design comes from the diversity of organic building blocks, stable geometry, and reversibility of dynamic covalent reactions. These materials are usually formed by organic building blocks consisting of heteroatoms and obtained in situ during the synthesis of 2/3D-ordered porous materials. The COFs are acclaimed as functional materials due to their crystallinity, porous nature, both chemical and thermal stability, low skeleton density, high surface area, diverse and easy synthetic methodologies, flexibility, insolubility, cheaper substrates, and highly simplified for functional modifications. Additionally, COFs can be used as appropriate carriers to lock metal ions or by using their functional in-built sites as a catalyst. This chapter summarizes the noteworthy progress in the synthesis of COFs material and their potential catalytic applications with an emphasis on their property as support material for heterogeneous catalysis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- COF:
-
Covalent organic framework
- CTF:
-
Covalent triazine framework
- MOF:
-
Metal–organic framework
- MNPs:
-
Metal nanoparticles
- CP-MAS:
-
Cross-polarization magic angle spinning
- NMR:
-
Nuclear magnetic resonance
- BDBA:
-
Benzene-1,4-diboronic acid
- PXRD:
-
Powder X-ray diffraction
- MCR:
-
Multi-component reactions
- DCM:
-
Dichloromethane
- DMF:
-
Dimethylformamide
- ORR:
-
Oxygen reduction reaction
- eV:
-
Electronvolt
- TFA:
-
Trifluoroacetic acid
- 2D/3D:
-
Two dimensional/three dimensional
- TON:
-
Turn over number
References
(a) Zhang Y, Riduan SN (2012) Functional porous organic polymers for heterogeneous catalysis. Chem Soc Rev 41(6):2083–2094; (b) Puthiaraj P, Lee Y-R, Zhang S, Ahn W-S (2016) Triazine-based covalent organic polymers: design, synthesis and applications in heterogeneous catalysis. J Mater Chem A 4(42):16288–16311
Fischbach DM, Rhoades G, Espy C, Goldberg F, Smith BJ (2019) Controlling the crystalline structure of imine-linked 3D covalent organic frameworks. Chem Commun 55(25):3594–3597
(a) Kuhn P, Antonietti M, Thomas A (2008) Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew Chem Int Ed 47(18):3450–3453. https://doi.org/10.1002/anie.200705710; (b) Roeser J, Kailasam K, Thomas A (2012) Covalent triazine frameworks as heterogeneous catalysts for the synthesis of cyclic and linear carbonates from carbon dioxide and epoxides. ChemSusChem 5(9):1793–1799
Zeng Y, Zou R, Zhao Y (2016) covalent organic frameworks for CO2 capture. Adv Mater 28(15):2855–2873
McKeown NB, Budd PM (2006) Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem Soc Rev 35(8):675–683
Cao H-L, Huang H-B, Chen Z, Karadeniz B, Lü J, Cao R (2017) Ultrafine silver nanoparticles supported on a conjugated microporous polymer as high-performance nanocatalysts for nitrophenol reduction. ACS Appl Mater Interfaces 9(6):5231–5236
Mülhaupt R (2004) Hermann staudinger and the origin of macromolecular chemistry. Angew Chem Int Ed 43(9):1054–1063
Côté AP, Benin AI, Ockwig NW, Keeffe M, Matzger AJ, Yaghi OM (2005) Porous, crystalline, covalent organic frameworks. Science 310(5751):1166
(a) Gomes R, Bhanja P, Bhaumik A (2015) A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem Commun 51(49):10050–10053; (b) Xiang Z, Zhou X, Zhou C, Zhong S, He X, Qin C, Cao D (2012) Covalent-organic polymers for carbon dioxide capture. J Mater Chem A 22(42):22663–22669
(a) Subodh, Prakash K, Masram DT (2020) A reversible chromogenic covalent organic polymer for gas sensing applications. Dalton Trans 49(4):1007–1010; (b) Subodh, Prakash K, Masram DT (2020) Chromogenic covalent organic polymer-based microspheres as solid-state gas sensor. J Mater Chem C 8(27):9201–9204
(a) Subodh, Prakash K, Chaudhary K, Masram DT (2020) A new triazine-cored covalent organic polymer for catalytic applications. Appl Catal A 593:117411; (b) Yadav D, Awasthi SK (2020) An unsymmetrical covalent organic polymer for catalytic amide synthesis. Dalton Trans 49(1):179–186
Shi Y, Fu Q, Li J, Liu H, Zhang Z, Liu T, Liu Z (2020) Covalent organic polymer as a carborane carrier for imaging-facilitated boron neutron capture therapy. ACS Appl Mater Interfaces 12(50):55564–55573
Patra BC, Khilari S, Manna RN, Mondal S, Pradhan D, Pradhan A, Bhaumik A (2017) A Metal-free covalent organic polymer for electrocatalytic hydrogen evolution. ACS Catal 7(9):6120–6127
Jejurkar VP, Yashwantrao G, Saha S (2020) Tröger’s base functionalized recyclable porous covalent organic polymer (COP) for dye adsorption from water. New J Chem 44(28):12331–12342
Julkapli NM, Bagheri S (2015) Graphene supported heterogeneous catalysts: an overview. Int J Hydrogen Energy 40(2):948–979
Subodh, Mogha NK, Chaudhary K, Kumar G, Masram DT (2018) Fur-imine-functionalized graphene oxide-immobilized copper oxide nanoparticle catalyst for the synthesis of xanthene derivatives. ACS Omega 3(11):16377–16385
(a) Chaudhary K, Subodh, Prakash K, Mogha NK, Masram DT (2020) Fruit waste (Pulp) decorated CuO NFs as promising platform for enhanced catalytic response and its peroxidase mimics evaluation. Arab J Chem 13(4):4869–4881; (b) Kumar G, Mogha NK, Kumar M, Subodh, Masram DT (2020) NiO nanocomposites/rGO as a heterogeneous catalyst for imidazole scaffolds with applications in inhibiting the DNA binding activity. Dalton Trans 49(6):1963–1974
Subodh, Chaudhary K, Prakash K, Masram DT (2020) TiO2 nanoparticles immobilized organo-reduced graphene oxide hybrid nanoreactor for catalytic applications. Appl Surf Sci 509:144902
(a) Liu Y, Zhou W, Teo WL, Wang K, Zhang L, Zeng Y, Zhao Y (2020) Covalent-organic-framework-based composite materials. Chem 6(12):3172–3202; (b) Yadav D, Awasthi SK (2020) A Pd NP-confined novel covalent organic polymer for catalytic applications. New J Chem 44(4):1320–1325
Hu H, Yan Q, Ge R, Gao Y (2018) Covalent organic frameworks as heterogeneous catalysts. Chin J Catal 39(7):1167–1179
Yadav D, Dixit AK, Raghothama S, Awasthi SK (2020) Ni nanoparticle-confined covalent organic polymer directed diaryl-selenides synthesis. Dalton Trans 49(35):12266–12272
Ren S, Bojdys MJ, Dawson R, Laybourn A, Khimyak YZ, Adams DJ, Cooper AI (2012) Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv Mater 24(17):2357–2361
Campbell NL, Clowes R, Ritchie LK, Cooper AI (2009) Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem Mater 21(2):204–206
Ji W, Guo Y-S, Xie H-M, Wang X, Jiang X, Guo D-S (2020) Rapid microwave synthesis of dioxin-linked covalent organic framework for efficient micro-extraction of perfluorinated alkyl substances from water. J Hazard Mater 397:122793
Yang S-T, Kim J, Cho H-Y, Kim S, Ahn W-S (2012) Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv 2(27):10179–10181
Biswal BP, Chandra S, Kandambeth S, Lukose B, Heine T, Banerjee R (2013) Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J Am Chem Soc 135(14):5328–5331
Mu M, Wang Y, Qin Y, Yan X, Li Y, Chen L (2017) Two-Dimensional imine-linked covalent organic frameworks as a platform for selective oxidation of olefins. ACS Appl Mater Interfaces 9(27):22856–22863
Bai L, Phua SZF, Lim WQ, Jana A, Luo Z, Tham HP, Zhao L, Gao Q, Zhao Y (2016) Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem Commun 52(22):4128–4131
Dinari M, Hatami M (2019) Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J Environ Chem Eng 7(1):102907
Wang P-L, Ding S-Y, Zhang Z-C, Wang Z-P, Wang W (2019) Constructing robust covalent organic frameworks via multicomponent reactions. J Am Chem Soc 141(45):18004–18008
Acharjya A, Longworth-Dunbar L, Roeser J, Pachfule P, Thomas A (2020) Synthesis of vinylene-linked covalent organic frameworks from acetonitrile: combining cyclotrimerization and aldol condensation in one pot. J Am Chem Soc 142(33):14033–14038
Wang K, Jia Z, Bai Y, Wang X, Hodgkiss SE, Chen L, Chong SY, Wang X, Yang H, Xu Y, Feng F, Ward JW, Cooper AI (2020) Synthesis of stable thiazole-linked covalent organic frameworks via a multicomponent reaction. J Am Chem Soc 142(25):11131–11138
Lin G, Gao C, Zheng Q, Lei Z, Geng H, Lin Z, Yang H, Cai Z (2017) Room-temperature synthesis of core–shell structured magnetic covalent organic frameworks for efficient enrichment of peptides and simultaneous exclusion of proteins. Chem Commun 53(26):3649–3652
He S, Zeng T, Wang S, Niu H, Cai Y (2017) Facile synthesis of magnetic covalent organic framework with three-dimensional bouquet-like structure for enhanced extraction of organic targets. ACS Appl Mater Interfaces 9(3):2959–2965
Wang R, Chen Z (2017) A covalent organic framework-based magnetic sorbent for solid phase extraction of polycyclic aromatic hydrocarbons, and its hyphenation to HPLC for quantitation. Microchim Acta 184(10):3867–3874
Lim H, Cha MC, Chang JY (2012) Preparation of microporous polymers based on 1,3,5-Triazine units showing high CO2 adsorption capacity. Macromol Chem Phys 213(13):1385–1390
Xiong S, Fu X, Xiang L, Yu G, Guan J, Wang Z, Du Y, Xiong X, Pan C (2014) Liquid acid-catalysed fabrication of nanoporous 1,3,5-triazine frameworks with efficient and selective CO2 uptake. Polym Chem 5(10):3424–3431
(a) Tian Y, Meng X, Duan J-y, Shi L (2012) A novel application of methanesulfonic acid as catalyst for the alkylation of olefins with aromatics. Ind Eng Chem Res 51(42):13627–13631; (b) Gernon MD, Wu M, Buszta T, Janney P (1999) Environmental benefits of methanesulfonic acid . comparative properties and advantages. Green Chem 1(3):127–140
Ren S, Dawson R, Laybourn A, Jiang J-X, Khimyak Y, Adams DJ, Cooper AI (2012) Functional conjugated microporous polymers: from 1,3,5-benzene to 1,3,5-triazine. Polym Chem 3(4):928–934
Mizuno N, Misono M (1998) Heterogeneous catalysis. Chem Rev 98(1):199–218
Kaleeswaran D, Antony R, Sharma A, Malani A, Murugavel R (2017) Catalysis and CO2 capture by palladium-incorporated covalent organic frameworks. ChemPlusChem 82(10):1253–1265
Tahir N, Wang G, Onyshchenko I, De Geyter N, Leus K, Morent R, Van Der Voort P (2019) High-nitrogen containing covalent triazine frameworks as basic catalytic support for the Cu-catalyzed Henry reaction. J Catal 375:242–248
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18(4):309–318
Balasubramanian P, Balamurugan TST, Chen S-M, Chen T-W (2019) Simplistic synthesis of ultrafine CoMnO3 nanosheets: an excellent electrocatalyst for highly sensitive detection of toxic 4-nitrophenol in environmental water samples. J Hazard Mater 361:123–133
Ansari A, Badhe RA, Garje SS (2019) Preparation of CdS–TiO2-based palladium heterogeneous nanocatalyst by solvothermal route and its catalytic activity for reduction of nitroaromatic compounds. ACS Omega 4(12):14937–14946
Fan M, Wang WD, Zhu Y, Sun X, Zhang F, Dong Z (2019) Palladium clusters confined in triazinyl-functionalized COFs with enhanced catalytic activity. Appl Catal B 257:117942
Yadav D, Awasthi SK (2020) A Pd confined hierarchically conjugated covalent organic polymer for hydrogenation of nitroaromatics: catalysis, kinetics, thermodynamics and mechanism. Green Chem 22(13):4295–4303
Zhang J, Han X, Wu X, Liu Y, Cui Y (2017) Multivariate chiral covalent organic frameworks with controlled crystallinity and stability for asymmetric catalysis. J Am Chem Soc 139(24):8277–8285
Ma H-C, Kan J-L, Chen G-J, Chen C-X, Dong Y-B (2017) Pd NPs-loaded homochiral covalent organic framework for heterogeneous asymmetric catalysis. Chem Mater 29(15):6518–6524
Zhang J, Han X, Wu X, Liu Y, Cui Y (2019) Chiral DHIP- and pyrrolidine-based covalent organic frameworks for asymmetric catalysis. ACS Sustain Chem Eng 7(5):5065–5071
Siriwardane RV, Shen M-S, Fisher EP (2003) Adsorption of CO2, N2, and O2 on natural zeolites. Energy Fuels 17(3):571–576
Lenden P, Ylioja PM, González-Rodríguez C, Entwistle DA, Willis MC (2011) Replacing dichloroethane as a solvent for rhodium-catalysed intermolecular alkyne hydroacylation reactions: the utility of propylene carbonate. Green Chem 13(8):1980–1982
(a) Decortes A, Castilla AM, Kleij AW (2014) Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew Chem Int Ed 49(51):9822–9837; (b) Kim SH, Ahn D, Go MJ, Park MH, Kim M, Lee J, Kim Y (2014) Dinuclear aluminum complexes as catalysts for cycloaddition of CO2 to epoxides. Organometallics 33(11):2770–2775
(a) Huang J-W, Shi M (2003) Chemical fixation of carbon dioxide by NaI/PPh3/PhOH. J Org Chem 68(17):6705–6709; (b) Yamaguchi K, Ebitani K, Yoshida T, Yoshida H, Kaneda K (1999) Mg–Al mixed oxides as highly active acid–base catalysts for cycloaddition of carbon dioxide to epoxides. J Am Chem Soc 121(18):4526–4527; (c) Yasuda H, He L-N, Takahashi T, Sakakura T (2006) Non-halogen catalysts for propylene carbonate synthesis from CO2 under supercritical conditions. Appl Catal A 298:177–180; (d) Kim HS, Kim JJ, Lee SD, Lah MS, Moon D, Jang HG (2003) New mechanistic insight into the coupling reactions of CO2 and epoxides in the presence of zinc complexes. Chem Eur J 9(3):678–686
Verma S, Kumar G, Ansari A, Kureshy RI, Khan N-UH (2017) A nitrogen rich polymer as an organo-catalyst for cycloaddition of CO2 to epoxides and its application for the synthesis of polyurethane. Sustain Energy Fuels 1(7):1620–1629
Liu T-T, Xu R, Yi J-D, Liang J, Wang X-S, Shi P-C, Huang Y-B, Cao R (2018) Imidazolium-based cationic covalent triazine frameworks for highly efficient cycloaddition of carbon dioxide. ChemCatChem 10(9):2036–2040
Ramón DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed 44(11):1602–1634
Zare E, Rafiee Z (2020) Magnetic chitosan supported covalent organic framework/copper nanocomposite as an efficient and recoverable catalyst for the unsymmetrical hantzsch reaction. J Taiwan Inst Chem Eng 116:205–214
Yao B-J, Wu W-X, Ding L-G, Dong Y-B (2021) Sulfonic acid and ionic liquid functionalized covalent organic framework for efficient catalysis of the Biginelli reaction. J Org Chem 86(3):3024–3032
Fang Q, Gu S, Zheng J, Zhuang Z, Qiu S, Yan Y (2014) 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew Chem 126(11):2922–2926
Sun Q, Aguila B, Ma S (2017) A bifunctional covalent organic framework as an efficient platform for cascade catalysis. Mater Chem Front 1(7):1310–1316
Kundu SK, Bhaumik A (2015) A triazine-based porous organic polymer: a novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes. RSC Adv 5(41):32730–32739
Cui Y, Du J, Liu Y, Yu Y, Wang S, Pang H, Liang Z, Yu J (2018) Design and synthesis of a multifunctional porous N-rich polymer containing s-triazine and Tröger’s base for CO2 adsorption, catalysis and sensing. Polym Chem 9(19):2643–2649
Yadav D, Awasthi S-K (2021) Ni nanoparticle-immobilized imine-linked microspherical covalent organic polymer for degradation studies of organic dyes. ACS Appl Polym Mater 3(11):5460–5469
Xu N, Wang R-L, Li D-P, Meng X, Mu J-L, Zhou Z-Y, Su Z-M (2018) A new triazine-based covalent organic polymer for efficient photodegradation of both acidic and basic dyes under visible light. Dalton Trans 47(12):4191–4197
Xu C, Xie Q, Zhang W, Xiong S, Pan C, Tang J, Yu G (2020) A vinylene-bridged conjugated covalent triazine polymer as a visible-light-active photocatalyst for degradation of methylene blue. Macromol Rapid Commun 41(7):2000006
Zhi Y, Li Z, Feng X, Xia H, Zhang Y, Shi Z, Mu Y, Liu X (2017) Covalent organic frameworks as metal-free heterogeneous photocatalysts for organic transformations. J Mater Chem A 5(44):22933–22938
Yang Y, Niu H, Xu L, Zhang H, Cai Y (2020) Triazine functionalized fully conjugated covalent organic framework for efficient photocatalysis. Appl Catal B 269:118799
Ma W, Yu P, Ohsaka T, Mao L (2015) An efficient electrocatalyst for oxygen reduction reaction derived from a Co-porphyrin-based covalent organic framework. Electrochem commun 52:53–57
Yao C-L, Li J-C, Gao W, Jiang Q (2018) An integrated design with new metal-functionalized covalent organic frameworks for the effective electroreduction of CO2. Chem Eur J 24(43):11051–11058
Royuela S, Martínez-Periñán E, Arrieta MP, Martínez JI, Ramos MM, Zamora F, Lorenzo E, Segura JL (2020) Oxygen reduction using a metal-free naphthalene diimide-based covalent organic framework electrocatalyst. Chem Commun 56(8):1267–1270
(a) Vardhan H, Hou L, Yee E, Nafady A, Al-Abdrabalnabi MA, Al-Enizi AM, Pan Y, Yang Z, Ma S (2019) Vanadium docked covalent-organic frameworks: an effective heterogeneous catalyst for modified Mannich-type reaction. ACS Sustain Chem Eng 7 (5):4878–4888; (b) Vardhan H, Verma G, Ramani S, Nafady A, Al-Enizi AM, Pan Y, Yang Z, Yang H, Ma S (2019) Covalent organic framework decorated with vanadium as a new platform for Prins reaction and sulfide oxidation. ACS Appl Mater Interfaces 11(3):3070–3079
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Subodh, Masram, D.T. (2022). Recent Advances in the Synthesis of Covalent Organic Frameworks for Heterogeneous Catalysis. In: Gulati, S. (eds) Metal-Organic Frameworks (MOFs) as Catalysts. Springer, Singapore. https://doi.org/10.1007/978-981-16-7959-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-16-7959-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7958-2
Online ISBN: 978-981-16-7959-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)