Abstract
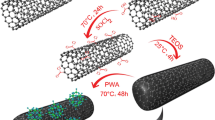
The functionalized nanosilicone particles with high sulfonic and carboxyl groups were prepared and incorporated into chitosan matrix to fabricate the doped biodegradable eco-friendly electrolyte materials. The functionalized nanosilicone particles not only were as crosslinkers and proton conductors, but also could form the compact silicone network. Compared with the undoped membrane, the doped ones showed better water retention, methanol barrier, thermal and mechanical stability. In particular, lower methanol uptake and diffusion in higher methanol concentration were very advantageous for reducing fuel wastage and increasing cell efficiency. The lowest methanol diffusion coefficient obtained in 12 M methanol (5.60 × 10−8 cm2 s−1) was less than 1/56 of that of Nafion® 117. In addition, proton conductivity of the doped membranes was significantly affected by nanosilicone particles content. The highest conductivity value obtained with 30% nanosilicone particles was 0.074 S cm−1 at 80 °C, which was very close to the value of Nafion® 117 at same conditions. The decreased methanol diffusion and improved conductivity conferred the super selectivity. In our work, the maximum selectivity was 5.30 × 105 Ss cm−3 which was approximately 33.5 times of that of Nafion® 117, indicating that the doped membranes were promising candidates for proton exchange membrane applications. Furthermore, the addition of functionalized nanosilicone particles endowed the membranes with many other advantages, including cost-effectiveness and simple preparation, which also improved the application potential of natural polymer chitosan as direct methanol fuel cell membrane material.









Similar content being viewed by others
References
Zhong S, Sun C, Luo Y, Liu W, Dou S (2013) Polymer electrolyte membranes with high selectivity based on silicon-containing sulfonated polystyrene/acrylate, poly(vinyl alcohol) and poly(2-acrylamido-2-methyl-1-propanesulfonic acid). J Power Sources 238:485–491
Wang M, Liu G, Cui X, Feng Y, Zhang H, Wang G, Zhong S, Luo Y (2018) Self-crosslinked organic-inorganic nanocomposite membranes with good methanol barrier for direct methanol fuel cell applications. Solid State Ion 315:71–76
Prapainainar P, Du Z, Kongkachuichay P, Holmes SM, Prapainainar C (2017) Mordenite/Nafion and analcime/Nafion composite membranes prepared by spray method for improved direct methanol fuel cell performance. Appl Surf Sci 421:24–41
Zhong S, Cui X, Gao Y, Liu W, Dou S (2014) Fabrication and properties of poly(vinyl alcohol)-based polymer electrolyte membranes for direct methanol fuel cell applications. Int J Hydrogen Energy 39:17857–17864
Uma Devi A, Divya K, Rana D, Sri Abirami Saraswathi M, Nagendran A (2018) Highly selective and methanol resistant polypyrrole laminated SPVdF-co-HFP/PWA proton exchange membranes for DMFC applications. Mater Chem Phys 212:533–542
Agudelo NA, Palacio J, López BL (2019) Effect of the preparation method on the morphology and proton conductivity of membranes based on sulfonated ABA triblock copolymers. J Mater Sci 54:4135–4153. https://doi.org/10.1007/s10853-018-3115-5
Zhong S, Sun C, Gao Y, Cui X (2015) Preparation and characterization of polymer electrolyte membranes based on silicon-containing core-shell structured nanocomposite latex particles. J Power Sources 289:34–40
Nagar H, Basava Rao VV, Sridhar S (2017) Synthesis and characterization of Torlon-based polyion complex for direct methanol and polymer electrolyte membrane fuel cells. J Mater Sci 52:8052–8069. https://doi.org/10.1007/s10853-017-1008-7
Parthiban V, Akula S, Peera SG, Islam N, Sahu AK (2016) Proton conducting Nafion-Sulfonated Graphene hybrid membranes for direct methanol fuel cells with reduced methanol crossover. Energy Fuels 30:725–734
Iulianelli A, Basile A (2012) Sulfonated PEEK-based polymers in PEMFC and DMFC applications: a review. Int J Hydrogen Energy 37:15241–15255
Wang J, Jiang H, Xu Y, Yang J, He R (2018) Quaternized poly(aromatic ether sulfone) with siloxane crosslinking networks as high temperature proton exchange membranes. Appl Surf Sci 452:473–480
Zhong S, Cui X, Dou S, Liu W, Gao Y, Hong B (2010) Improvement in silicon-containing sulfonated polystyrene/acrylate membranes by blending and crosslinking. Electrochim Acta 55:8410–8415
Lee C, Mu Jo S, Choi J, Baek KY, Truong YB, Kyratzis IL, Shul YG (2013) SiO2/sulfonated poly ether ether ketone (SPEEK) composite nanofiber mat supported proton exchange membranes for fuel cells. J Mater Sci 48:3665–3671. https://doi.org/10.1007/s10853-013-7162-7
Cui X, Guan X, Zhong S, Chen J, Zhu H, Li Z, Xu F, Chen P, Wang H (2017) Multi-stimuli responsive smart chitosan-based microcapsules for targeted drug delivery and triggered drug release. Ultrason Sonochem 38:145–153
Sahariah P, Másson M (2017) Antimicrobial chitosan and chitosan derivatives: a review of the structure–activity relationship. Biomacromolecules 18:3846–3868
Zhong S, Zhang H, Liu Y, Wang G, Shi C, Li Z, Feng Y, Cui X (2017) Folic acid functionalized reduction-responsive magnetic chitosan nanocapsules for targeted delivery and triggered release of drugs. Carbohydr Polym 168:282–289
Xu P, Cao X, Tang J, Gao J, Liang J, Chen Y, Gao Q, Zhang R, Shao W (2016) Facile synthesis of Ag-deposited macroporous magnetic chitosan spheres for antibacterial application. J Nanosci Nanotechnol 16:6847–6853
Wang H, Qian J, Ding F (2018) Emerging chitosan-based films for food packaging applications. J Agric Food Chem 66:395–413
Zhang D, Xiao J, Guo Q, Yang J (2019) 3D-printed highly porous and reusable chitosan monoliths for Cu(II) removal. J Mater Sci 54:6728–6741. https://doi.org/10.1007/s10853-019-03332-y
Xu F, Zhao T, Yang T, Dong L, Guan X, Cui X (2016) Fabrication of folic acid functionalized pH-responsive and thermosensitive magnetic chitosan microcapsules via a simple sonochemical method. Colloids Surf A 490:22–29
Xu P, Liang X, Chen N, Tang J, Shao W, Gao Q, Teng Z (2017) Magnetic separable chitosan microcapsules decorated with silver nanoparticles for catalytic reduction of 4-nitrophenol. J Colloid Interface Sci 507:353–359
Shanta P, Paras NY (2019) Functionalization of chitosan polymer and their applications. J Macromo. Sci Part A Pure Appl. Chem 56:450–475
Shirdast A, Sharif A, Abdollahi M (2016) Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J Power Sources 306:541–551
Santamaria M, Pecoraro CM, Quarto FD, Bocchetta P (2015) Chitosan-phosphotungstic acid complex as membranes for low temperature H2-O2 fuel cell. J Power Sources 276:189–194
Wang W, Shan B, Zhu L, Xie C, Liu C, Cui F (2018) Anatase titania coated CNTs and sodium lignin sulfonate doped chitosan proton exchange membrane for DMFC application. Carbohydr Polym 187:35–42
Shaari N, Kamarudin SK (2015) Chitosan and alginate types of bio-membrane in fuel cell application: an overview. J Power Sources 289:71–80
Tracy CA, Adler AM, Nguyen A, Johnson RD, Miller KM (2018) Covalently crosslinked 1,2,3-triazolium-containing polyester networks: thermal, mechanical, and conductive properties. ACS Omega 3:13442–13453
Meemuk C, Chirachanchai S (2018) Stable and effective proton exchange membrane formation via cross-linking the polymeric proton donor and proton acceptor in a layer-by-layer structure. Int J Hydrogen Energy 43:6701–6710
Özdemir Y, Özkan N, Devrim Y (2017) Fabrication and characterization of cross-linked polybenzimidazole based membranes for high temperature PEM fuel cells. Electrochim Acta 245:1–13
Cui X, Zhong S, Wang H (2007) Organic-inorganic hybrid proton exchange membranes based on silicon-containing polyacrylate nanoparticles with phosphotungstic acid. J Power Sources 173:28–35
Palani PB, Abidin KS, Kannan R, Sivakumar M, Wang FM, Rajashabala S, Velraj G (2014) Improvement of proton conductivity in nanocomposite polyvinyl alcohol (PVA)/chitosan (CS) blend membranes. RSC Adv 4:61781–61789
Vijayalekshmi V, Khastgir D (2017) Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J Membr Sci 523:45–59
Yang J, Chiu H (2012) Preparation and characterization of polyvinyl alcohol/chitosan blended membrane for alkaline direct methanol fuel cells. J Membr Sci 419–420:65–71
Bai H, Li Y, Zhang H, Chen H, Wu W, Wang J, Liu J (2015) Anhydrous proton exchange membranes comprising of chitosan and phosphorylated graphene oxide for elevated temperature fuel cells. J Membr Sci 495:48–60
Wang J, Zheng X, Wu H, Zheng B, Jiang Z, Hao X, Wang B (2008) Effect of zeolites on chitosan/zeolite hybrid membranes for direct methanol fuel cell. J Power Sources 178:9–19
Chen W, Kuo P (2007) Covalently cross-Linked perfluorosulfonated membranes with polysiloxane framework. Macromolecules 40:1987–1994
Jiang Z, Zheng X, Wu H, Wang J, Wang Y (2008) Proton conducting CS/P(AA-AMPS) membrane with reduced methanol permeability for DMFCs. J Power Sources 180:143–153
Acknowledgements
This work was financially supported by the Natural Science Foundation of the Science and Technology Department of Jilin Province (20180101072JC), the Key Program of the Science and Technology Department of Jilin Province, PR China (No. 20180201082SF), the scientific research Program of the Education Department of Jilin Province (P. R. China) during the 13th Five-Year Plan Period (Nos. JJKH20180119KJ and JJKH20190914KJ), People’s Republic of China and National Training Programs of Innovation and Entrepreneurship for Undergraduates (No. 201810193023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Shang, J., Zhao, Y. et al. Fabrication of functionalized nanosilicone particles-doped biodegradable eco-friendly proton exchange membranes. J Mater Sci 54, 14504–14514 (2019). https://doi.org/10.1007/s10853-019-03962-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03962-2