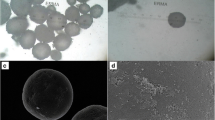
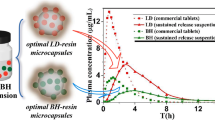
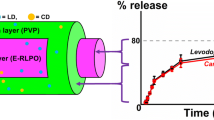
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder primarily affecting elderly patients. In order to efficiently manage the symptoms of the disease, controlled and continuous supply of PD drugs [levodopa (LD) and carbidopa (CD)] would be highly beneficial. Currently available commercial tablets are not sufficiently capable of releasing PD drugs in a sustainable fashion. Hence, the purpose of this study was to engineer a carrier system to deliver more than one therapeutic to the target site with independent, sustainable release kinetics. To achieve that, we have designed and developed a set of disk-shaped microparticles composed of two distinct compartments, the first containing a relatively hydrophilic amorphous polymer, poly(lactide-co-glycolide) and the second composed entirely of hydrophobic semicrystalline polylactide. These bicompartmental microparticles with narrow particle size distribution (coefficient of variance ~ 6%) were fabricated by electrohydrodynamic co-jetting technique and characterized by optical microscopy, confocal laser scanning microscopy, SEM, DSC and Raman microscopy. The feasibility of the bicompartmentalized microparticles was confirmed by localizing two different hydrophilic PD drugs (LD and CD) in two different compartments with 4:1 ratio similar to commercially available tablets. This one-step particle fabrication method generated bicompartmental particles with high drug encapsulation efficiency (LD: > 97% and CD: > 68%). The resulting dual drug-loaded disk-shaped microparticles demonstrated simultaneous release of two independent PD drugs with similar release pattern (> 80% release of both drugs within 5 h). These compartmentalized microparticles can potentially act as dual drug delivery system to enable controlled, sustained release of a wide range of water soluble drugs including PD drugs.







Similar content being viewed by others
References
Garbayo E, Ansorena E, Blanco-Prieto MJ (2013) Drug development in Parkinson’s disease: from emerging molecules to innovative drug delivery systems. Maturitas 76(3):272–278
Maysinger D, Jalsenjak V, Stolnik S, Jalsenjak I (1992) Release of l-Dopa from HSA microspheres into the rat brain: in vitro and in vivo characterization by microdialysis. In: Whateley TL (ed) Microencapsulation of drugs. Harwood Academic Publication, Chur, pp 269–275
During MJ, Freese A, Sabel BA, Saltzman WM, Deutch A, Roth RH, Langer R (1989) Controlled release of dopamine from a polymeric brain implant: in vivo characterization. Ann Neurol 25(4):351–356
Vallbacka JJ, Nobrega JN, Sefton MV (2001) Tissue engineering as a platform for controlled release of therapeutic agents: implantation of microencapsulated dopamine producing cells in the brains of rats. J Control Release 72(1):93–100
Bianchine JR, McGhee B (1985) MPTP and parkinsonism. Ration Drug Ther 19(2):5–7
Hr A, Michel P, Giovanni A, Linda M, Nalina D, Algirdas K (2009) Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord 24(4):541–550
Mathers SE, Kempster PA, Swash M, Lees AJ (1988) Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry 51(12):1503–1507
Buckley G (1989) Martindale: the extra pharmacopoeia (29th edition). J R Coll Gen Pract 39(327):440
Seeberger LC, Hauser RA (2009) Levodopa/carbidopa/entacapone in Parkinson’s disease. Expert Rev Neurother 9(7):929–940
Sharma S, Lohan S, Murthy RSR (2014) Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev Ind Pharm 40(7):869–878
Arıca B, Kaş HS, Moghdam A, Akalan N, Hıncal AA (2005) Carbidopa/levodopa-loaded biodegradable microspheres: in vivo evaluation on experimental Parkinsonism in rats. J Control Release 102(3):689–697
Baek J-S, Choo CC, Qian C, Tan NS, Shen Z, Loo SCJ (2016) Multi-drug-loaded microcapsules with controlled release for management of Parkinson’s disease. Small 12(27):3712–3722
Parhizkar M, Reardon PJT, Knowles JC, Browning RJ, Stride E, Barbara PR, Harker AH, Edirisinghe M (2016) Electrohydrodynamic encapsulation of cisplatin in poly (lactic-co-glycolic acid) nanoparticles for controlled drug delivery. Nanomed Nanotechnol Biol Med 12(7):1919–1929
Taylor G (1964) Disintegration of water drops in an electric field. Proc R Soc A Math Phys Eng Sci 280(1382):383–397
Bhaskar S, Lahann J (2009) Microstructured materials based on multicompartmental fibers. J Am Chem Soc 131(19):6650–6651
Roh K-H, Martin DC, Lahann J (2006) Triphasic nanocolloids. J Am Chem Soc 128(21):6796–6797
Bhaskar S, Hitt J, Chang SW, Lahann J (2009) Multicompartmental microcylinders. Angew Chem 48(25):4589–4593 (international ed in English)
Roh KH, Martin DC, Lahann J (2005) Biphasic Janus particles with nanoscale anisotropy. Nat Mater 4(10):759–763
Bhaskar S, Pollock KM, Yoshida M, Lahann J (2010) Towards designer microparticles: simultaneous control of anisotropy, shape, and size. Small 6(3):404–411
Lee KJ, Yoon J, Rahmani S, Hwang S, Bhaskar S, Mitragotri S, Lahann J (2012) Spontaneous shape reconfigurations in multicompartmental microcylinders. Proc Natl Acad Sci 109(40):16057–16062. https://doi.org/10.1073/pnas.1213669109
Naraharisetti PK, Ning Lew MD, Fu Y-C, Lee D-J, Wang C-H (2005) Gentamicin-loaded discs and microspheres and their modifications: characterization and in vitro release. J Control Release 102(2):345–359
Ahmed A, Bonner C, Desai TA (2002) Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. J Control Release 81(3):291–306
Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S (2016) Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release 238:176–185. https://doi.org/10.1016/j.jconrel.2016.07.051
Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR (2012) The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv 3(2):181–194. https://doi.org/10.4155/tde.11.156
Fan J, Guo Y, Wang J, Fan M (2009) Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J Hazard Mater 166(2):904–910
Rao AV, Shiwnarain N, Maharaj I (1989) Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can Inst Food Sci Technol J 22(4):345–349
Marques M (2004) Dissolution media simulating fasted and fed states. Dissolut Technol 11:16–19
Sharma B (2017) Synthesis of fluorene and cyclopentadithiophene based donor-acceptor type low band-gap cross-conjugated polymers for solar cell applications. IIT, Delhi
Lee WL, Loei C, Widjaja E, Loo SCJ (2011) Altering the drug release profiles of double-layered ternary-phase microparticles. J Control Release 151(3):229–238
Kim K, Yu M, Zong X, Chiu J, Fang D, Seo Y-S, Hsiao BS, Chu B, Hadjiargyrou M (2003) Control of degradation rate and hydrophilicity in electrospun non-woven poly(d, l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 24(27):4977–4985
Park TG (1995) Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials 16(15):1123–1130
Bhaskar S, Roh K-H, Jiang X, Baker GL, Lahann J (2008) Spatioselective modification of bicompartmental polymer particles and fibers via huisgen 1,3-dipolar cycloaddition. Macromol Rapid Commun 29(20):1655–1660
Müller T, Erdmann C, Bremen D, Schmidt WE, Muhlack S, Woitalla D, Goetze O (2006) Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin Neuropharmacol 29(2):61–67
Koombhongse S, Liu W, Reneker DH (2001) Flat polymer ribbons and other shapes by electrospinning. J Polym Sci, Part B: Polym Phys 39(21):2598–2606
Xie J, Lim LK, Phua Y, Hua J, Wang C-H (2006) Electrohydrodynamic atomization for biodegradable polymeric particle production. J Colloid Interface Sci 302(1):103–112
Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S (2009) Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci 106(51):21495–21499
Arias V, Odelius K, Albertsson A-C (2014) Nano-stereocomplexation of polylactide (PLA) spheres by spray droplet atomization. Macromol Rapid Commun 35(22):1949–1953
Lahann J, Hwang SY, Yoon J, Bhaskar S, Lee K, Park TH (2016) Optical devices with switchable particles. Google Patents
Bhaskar S, Gibson CT, Yoshida M, Nandivada H, Deng X, Voelcker NH, Lahann J (2011) Engineering, characterization and directional self-assembly of anisotropically modified nanocolloids. Small 7(6):812–819
Rahmani S, Saha S, Durmaz H, Donini A, Misra AC, Yoon J, Lahann J (2014) Chemically orthogonal three-patch microparticles. Angew Chem Int Ed Engl 53(9):2332–2338
Lee WL, Widjaja E, Loo SCJ (2010) One-step fabrication of triple-layered polymeric microparticles with layer localization of drugs as a novel drug-delivery system. Small 6(9):1003–1011. https://doi.org/10.1002/smll.200901985
Bhaskar S (2011) Multicompartmental biomaterials via electrohydrodynamic co-jetting, chapter-9. PhD thesis, University of Michigan, Ann Arbor
Sungkapreecha C, Iqbal N, Gohn AM, Focke WW, Androsch R (2017) Phase behavior of the polymer/drug system PLA/DEET. Polymer 126(Supplement C):116–125
Edwin B, Hubert Joe I (2013) Vibrational spectral analysis of anti-neurodegenerative drug Levodopa: a DFT study. J Mol Struct 1034:119–127
Frazao NF, Albuquerque EL, Fulco UL, Mauriz PW, Azevedo DL (2013) Optoelectronics and vibrational properties of carbidopa from quantum chemistry computations. Indian Ocean Rev Sci Technol 1:1–12
Sukker GM, Wazzan N, Hilaal R (2015) Towards understanding mode of action of L-Dopa and carbidopa: DFT/TD-DFT analyse of their electronic and vibrational spectra. Indian J Chem 54A:1378–1386
Mittur A, Gupta S, Modi NB (2017) Pharmacokinetics of Rytary®, an extended-release capsule formulation of carbidopa–levodopa. Clin Pharmacokinet 56(9):999–1014
F-d Cui, A-j Tao, D-m Cun, L-q Zhang, Shi K (2007) Preparation of insulin loaded PLGA-Hp55 nanoparticles for oral delivery. J Pharm Sci 96(2):421–427
Feng S-S, Mei L, Anitha P, Gan CW, Zhou W (2009) Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of docetaxel. Biomaterials 30(19):3297–3306
Italia JL, Bhatt DK, Bhardwaj V, Tikoo K, Kumar MNVR (2007) PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral®. J Control Release 119(2):197–206
Tobío M, Sánchez A, Vila A, Soriano I, Evora C, Vila-Jato JL, Alonso MJ (2000) The role of PEG on the stability in digestive fluids and in vivo fate of PEG–PLA nanoparticles following oral administration. Colloids Surf B 18(3):315–323
Foss AC, Goto T, Morishita M, Peppas NA (2004) Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm 57(2):163–169
Hu Y, Jiang X, Ding Y, Ge H, Yuan Y, Yang C (2002) Synthesis and characterization of chitosan–poly(acrylic acid) nanoparticles. Biomaterials 23(15):3193–3201
Ensign LM, Cone R, Hanes J (2012) Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev 64(6):557–570
Khonsari F, Zakeri-Milani P, Jelvehgari M (2014) Formulation and Evaluation of In-vitro Characterization of Gastic-Mucoadhesive Microparticles/Discs Containing Metformin Hydrochloride. Iran J Pharm Res IJPR 13(1):67–80
Lennernas H (2007) Modeling gastrointestinal drug absorption requires more in vivo biopharmaceutical data: experience from in vivo dissolution and permeability studies in humans. Curr Drug Metab 8(7):645–657
Thwaites DT, Anderson CMH (2007) H + -coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92(4):603–619
Tang Y-D, Venkatraman SS, Boey FYC, Wang L-W (2007) Sustained release of hydrophobic and hydrophilic drugs from a floating dosage form. Int J Pharm 336(1):159–165
Liu Y, Zhang J, Gao Y, Zhu J (2011) Preparation and evaluation of glyceryl monooleate-coated hollow-bioadhesive microspheres for gastroretentive drug delivery. Int J Pharm 413(1):103–109
Drugbank Levodopa and Carbidopa. https://www.drugbank.ca/drugs/DB01235, https://www.drugbank.ca/drugs/DB00190
Martini LG, Avontuur P, George A, Willson RJ, Crowley PJ (1999) Solubility parameter and oral absorption. Eur J Pharm Biopharm 48(3):259–263
Liu H, Finn N, Yates MZ (2005) Encapsulation and sustained release of a model drug, indomethacin, using CO2-based microencapsulation. Langmuir 21(1):379–385
Siepmann J, Peppas NA (2001) Mathematical modeling of controlled drug delivery. Adv Drug Deliv Rev 48(2–3):137–138
Young CR, Dietzsch C, Cerea M, Farrell T, Fegely KA, Rajabi-Siahboomi A, McGinity JW (2005) Physicochemical characterization and mechanisms of release of theophylline from melt-extruded dosage forms based on a methacrylic acid copolymer. Int J Pharm 301(1):112–120
Li Y, Li H, Wei M, Lu J, Jin L (2009) pH-Responsive composite based on prednisone-block copolymer micelle intercalated inorganic layered matrix: structure and in vitro drug release. Chem Eng J 151(1):359–366
Glaive A-S, Modjinou T, Versace D-L, Abbad-Andaloussi S, Dubot P, Langlois V, Renard E (2017) Design of antibacterial and sustainable antioxidant networks based on plant phenolic derivatives used as delivery system of carvacrol or tannic acid. ACS Sustain Chem Eng 5(3):2320–2329
Gouda R, Baishya H, Qing Z (2017) Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J Dev Drugs 6:171
Colombo P, Bettini R, Catellani PL, Santi P, Peppas NA (1999) Drug volume fraction profile in the gel phase and drug release kinetics in hydroxypropylmethyl cellulose matrices containing a soluble drug. Eur J Pharm Sci 9(1):33–40
Acknowledgements
The research leading to these results has received funding from Department of Science and Technology (DST), New Delhi, India, under Extramural Research Grant: SB/S3/CE/068/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parthipan, A.K., Gupta, N., Pandey, K. et al. One-step fabrication of bicompartmental microparticles as a dual drug delivery system for Parkinson’s disease management. J Mater Sci 54, 730–744 (2019). https://doi.org/10.1007/s10853-018-2819-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2819-x