Abstract
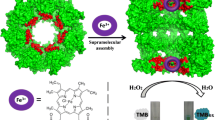
Supramolecular anchoring of metalloporphyrins in a protein is an attractive approach to the generation of artificial enzymes. Here, we employ the hydrophobic nanocage of single-ring mutant of bacterial GroEL protein for this purpose. We found that multiple monomeric hemin cofactors can be efficiently loaded into the protein nanocage. The as-prepared biohybrid possessed an oxidase-like catalytic activity and followed the typical Michaelis–Menten kinetics and a ping-pong mechanism in the H2O2-mediated oxidation of model substrates. In comparison with natural peroxidase, the artificial enzyme exhibited higher affinity for the model substrate. A simple and sensitive colorimetric method for the quantitative detection of H2O2 and glucose was also developed based on the artificial enzyme, with the detection limits determined to be 3.0 μM for H2O2 and 5.0 μM for glucose, respectively. The protein nanocage-based artificial enzyme is very flexible and is envisioned to be adapted readily for binding other metal complexes and catalysis of other reactions.






Similar content being viewed by others
References
Durrenberger M, Ward TR (2014) Recent achievments in the design and engineering of artificial metalloenzymes. Curr Opin Chem Biol 19:99–106
Hocker B (2012) Protein design: a metalloenzyme reloaded. Nat Chem Biol 8(3):224–225
Jeschek M, Reuter R, Heinisch T, Trindler C, Klehr J, Panke S, Ward TR (2016) Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537(7622):661–665
Lin YH, Ren JS, Qu XG (2014) Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res 47(4):1097–1105
Yu FT, Cangelosi VM, Zastrow ML, Tegoni M, Plegaria JS, Tebo AG, Mocny CS, Ruckthong L, Qayyum H, Pecoraro VL (2014) Protein design: toward functional metalloenzymes. Chem Rev 114(7):3495–3578
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Andre R, Natalio F, Humanes M, Leppin J, Heinze K, Wever R, Schroder HC, Muller WEG, Tremel W (2011) V2O5 nanowires with an intrinsic peroxidase-like activity. Adv Funct Mater 21(3):501–509
Jampaiah D, Reddy TS, Kandjani AE, Selvakannan PR, Sabri YM, Coyle VE, Shukla R, Bhargava SK (2016) Fe-doped CeO2 nanorods for enhanced peroxidase-like activity and their application towards glucose detection. J Mater Chem B 4(22):3874–3885
Li JN, Liu WQ, Wu XC, Gao XF (2015) Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 48:37–44
Liu J, Xin XY, Zhou H, Zhang SS (2015) A ternary composite based on graphene, hemin, and gold nanorods with high catalytic activity for the detection of cell-surface glycan expression. Chem Eur J 21(5):1908–1914
Luo WJ, Zhu CF, Su S, Li D, He Y, Huang Q, Fan CH (2010) Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano 4(12):7451–7458
Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R, Tremel W (2012) Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol 7(8):530–535
Song YJ, Qu KG, Zhao C, Ren JS, Qu XG (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22(19):2206–2210
Song YJ, Wang XH, Zhao C, Qu KG, Ren JS, Qu XG (2010) Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem Eur J 16(12):3617–3621
Sun HJ, Zhao AD, Gao N, Li K, Ren JS, Qu XG (2015) Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew Chem Int Edit 54(24):7176–7180
Tian T, Ai LH, Liu XM, Li LL, Li J, Jiang J (2015) Synthesis of hierarchical FeWO4 architectures with {100}-faceted nanosheet assemblies as a robust biomimetic catalyst. Ind Eng Chem Res 54(4):1171–1178
Zheng XX, Liu Q, Jing C, Li Y, Li D, Luo WJ, Wen YQ, He Y, Huang Q, Long YT, Fan CH (2011) Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angew Chem Int Edit 50(50):11994–11998
Albada HB, Golub E, Willner I (2016) Rational design of supramolecular hemin/G-quadruplex-dopamine aptamer nucleoapzyme systems with superior catalytic performance. Chem Sci 7(5):3092–3101
Mahy JP, Marechal JD, Ricoux R (2015) From “hemoabzymes” to “hemozymes”: towards new biocatalysts for selective oxidations. Chem Commun 51(13):2476–2494
Qu R, Shen LL, Chai ZH, Jing C, Zhang YF, An YL, Shi LQ (2014) Hemin-block copolymer micelle as an artificial peroxidase and its applications in chromogenic detection and biocatalysis. ACS Appl Mater Int 6(21):19207–19216
Wang QG, Yang ZM, Zhang XQ, Xiao XD, Chang CK, Xu B (2007) A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew Chem Int Edit 46(23):4285–4289
Bos J, Browne WR, Driessen AJM, Roelfes G (2015) Supramolecular assembly of artificial metalloenzymes based on the dimeric protein LmrR as promiscuous scaffold. J Am Chem Soc 137(31):9796–9799
Hayer-Hartl M, Bracher A, Hartl FU (2016) The GroEL–GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem Sci 41(1):62–76
Krainer FW, Glieder A (2015) An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Appl Microbiol Biotechnol 99(4):1611–1625
Bode SA, Minten IJ, Nolte RJ, Cornelissen JJ (2011) Reactions inside nanoscale protein cages. Nanoscale 3(6):2376–2389
Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, Varpness Z, Liepold LO, Young M, Douglas T (2007) Biological containers: protein cages as multifunctional nanoplatforms. Adv Mater 19(8):1025–1042
Witus LS, Francis MB (2011) Using synthetically modified proteins to make new materials. Acc Chem Res 44(9):774–783
Jutz G, van Rijn P, Miranda BS, Boker A (2015) Ferritin: a versatile building block for bionanotechnology. Chem Rev 115(4):1653–1701
Jordan PC, Patterson DP, Saboda KN, Edwards EJ, Miettinen HM, Basu G, Thielges MC, Douglas T (2016) Self-assembling biomolecular catalysts for hydrogen production. Nat Chem 8(2):179–185
Fiedler JD, Brown SD, Lau JL, Finn MG (2010) RNA-directed packaging of enzymes within virus-like particles. Angew Chem Int Edit 49(50):9648–9651
Weissman JS, Hohl CM, Kovalenko O, Kashi Y, Chen S, Braig K, Saibil HR, Fenton WA, Horwich AL (1995) Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell 83(4):577–587
Wang XQ, Wang C, Pan MH, Wei JT, Jiang FP, Lu RS, Liu X, Huang YH, Huang F (2017) Chaperonin-nanocaged hemin as an artificial metalloenzyme for oxidation catalysis. ACS Appl Mater Int 9(30):25387–25396
Chen Q, Chen J, Gao CJ, Zhang ML, Chen JY, Qiu HD (2015) Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst 140(8):2857–2863
Jurow M, Schuckman AE, Batteas JD, Drain CM (2010) Porphyrins as molecular electronic components of functional devices. Coord Chem Rev 254(19–20):2297–2310
Ryabova ES, Dikiy A, Hesslein AE, Bjerrum MJ, Ciurli S, Nordlander E (2004) Preparation and reactivity studies of synthetic microperoxidases containing b-type heme. J Biol Inorg Chem 9(4):385–395
Hitomi Y, Hiramatsu K, Arakawa K, Takeyasu T, Hata M, Kodera M (2013) An iron(III) tetradentate monoamido complex as a nonheme iron-based peroxidase mimetic. Dalton Trans 42(36):12878–12882
Porter DJ, Bright HJ (1983) The mechanism of oxidation of nitroalkanes by horseradish peroxidase. J Biol Chem 258(16):9913–9924
Cai SF, Han QS, Qi C, Lian Z, Jia XH, Yang R, Wang C (2016) Pt74Ag26 nanoparticle-decorated ultrathin MoS2 nanosheets as novel peroxidase mimics for highly selective colorimetric detection of H2O2 and glucose. Nanoscale 8(6):3685–3693
Zhao K, Gu W, Zheng SS, Zhang CL, Xian YZ (2015) SDS-MoS2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta 141:47–52
Lin TR, Zhong LS, Guo LQ, Fu FF, Chen GN (2014) Seeing diabetes: visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 6(20):11856–11862
Shi WB, Wang QL, Long YJ, Cheng ZL, Chen SH, Zheng HZ, Huang YM (2011) Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun 47(23):6695–6697
Mu JS, Wang Y, Zhao M, Zhang L (2012) Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem Commun 48(19):2540–2542
Qin FX, Jia SY, Wang FF, Wu SH, Song J, Liu Y (2013) Hemin@metal-organic framework with peroxidase-like activity and its application to glucose detection. Catal Sci Technol 3(10):2761–2768
Wei H, Wang E (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80(6):2250–2254
Zhang LH, Zhai YM, Gao N, Wen D, Dong SJ (2008) Sensing H2O2 with layer-by-layer assembled Fe3O4-PDDA nanocomposite film. Electrochem Commun 10(10):1524–1526
Chen Q, Liu ML, Zhao JN, Peng X, Chen XJ, Mi NX, Yin BD, Li HT, Zhang YY, Yao SZ (2014) Water-dispersible silicon dots as a peroxidase mimetic for the highly-sensitive colorimetric detection of glucose. Chem Commun 50(51):6771–6774
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21503278) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Xu, B. & Liu, Z. The oxidase-like activity of hemin encapsulated by single-ring GroEL mutant and its application for colorimetric detection. J Mater Sci 53, 8786–8794 (2018). https://doi.org/10.1007/s10853-018-2215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2215-6