Abstract
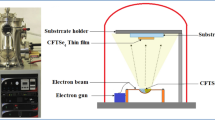
Copper chalcogenide materials are interesting for multiple applications due to the feasibility of suiting their optical absorption and electrical conduction by the creation of copper vacancies. Here, Cu x S and Cu x Se nanocrystalline films with p-type conductivity have been obtained by heating evaporated copper layers of various thicknesses with elemental sulfur or selenium, at temperatures ranging from 250 to 400 °C. These preparation parameters determine the composition and the crystalline structure of the samples, which in turn control their morphology, optical and electrical properties. Thus, the surface roughness increases with the mean crystallite size, whereas the hole concentration increases as the copper atomic proportion (or x value) decreases. Owing to the high carrier densities achieved, around 1022 cm−3, the samples show a metallic behavior with plasmonic absorption in the near infrared and electrical transport dominated by phonon scattering. Apart from such common behavior, some differences have been established between the sulfide and selenide films. One is the superior thermal stability of hexagonal CuS, present in all the temperature range, with respect to hexagonal CuSe, which evolves to cubic Cu1.8Se above 300 °C. Other is about the bandgap, wider for the sulfide than selenide samples.









Similar content being viewed by others
References
Kundu J, Pradhan D (2014) Controlled synthesis and catalytic activity of copper sulfide nanostructured assemblies with different morphologies. ACS Appl Mater Interfaces 6:1823–1834. doi:10.1021/am404829g
Sagade AA, Sharma R (2008) Copper sulphide (Cu x S) as an ammonia gas sensor working at room temperature. Sens Actuators B Chem 133:135–143. doi:10.1016/j.snb.2008.02.015
Chen YH, Davoisne C, Tarascon JM, Guery C (2012) Growth of single-crystal copper sulfide thin films via electrodeposition in ionic liquid media for lithium ion batteries. J Mater Chem 22:5295–5299. doi:10.1039/C2jm16692e
Aguilar JO, Gomez-Daza O, Brito LA et al (2005) Optical and mechanical characteristics of clear and solar control laminated glass using zinc sulphide and copper sulphide thin films. Surf Coat Technol 200:2557–2565. doi:10.1016/j.surfcoat.2004.10.140
Zhang H, Wang C, Peng W et al (2016) Quantum dot sensitized solar cells with efficiency up to 8.7% based on heavily copper-deficient copper selenide counter electrode. Nano Energy 23:60–69. doi:10.1016/j.nanoen.2016.03.009
Korala L, McGoffin JT, Prieto AL (2016) Enhanced conductivity in CZTS/Cu2−x Se nanocrystal thin films: growth of a conductive shell. ACS Appl Mater Interfaces 8:4911–4917. doi:10.1021/acsami.5b11037
Blachnik R, Müller A (2000) The formation of Cu2S from the elements: I. Copper used in form of powders. Thermochim Acta 361:31–52
Glazov VM, Pashinkin AS, Fedorov VA (2000) Phase equilibria in the Cu–Se system. Inorg Mater 36:641–652. doi:10.1007/BF02758413
Zhang Y, Wang Y, Xi L et al (2014) Electronic structure of antifluorite Cu2X (X = S, Se, Te) within the modified Becke–Johnson potential plus an on-site Coulomb U. J Chem Phys. doi:10.1063/1.4865257
Choi JH, Han YK (2015) Structural, electronic, and optical properties of bulk Cu2Se. Curr Appl Phys 15:1417–1420. doi:10.1016/j.cap.2015.08.006
Liu G, Schulmeyer T, Brötz J et al (2003) Interface properties and band alignment of Cu2S/CdS thin film solar cells. Thin Solid Films 431–432:477–482. doi:10.1016/S0040-6090(03)00190-1
Lukashev P, Lambrecht WRL, Kotani T, van Schilfgaarde M (2007) Electronic and crystal structure of Cu2−x S: full-potential electronic structure calculations. Phys Rev B 76:195202. doi:10.1103/PhysRevB.76.195202
Coughlan C, Ibáñez M, Dobrozhan O et al (2017) Compound copper chalcogenide nanocrystals. Chem Rev. doi:10.1021/acs.chemrev.6b00376
He Y, Polity A, Österreicher I (2001) Hall effect and surface characterization of Cu2S and CuS films deposited by RF reactive sputtering. Phys B Condens Matter 310:1069–1073
Wang L, Ren B, Weng L et al (2015) Metal-semiconductor transition in CuS–Cu1.8S mixed phase thin films. Vacuum 112:70–72. doi:10.1016/j.vacuum.2014.11.018
Comin A, Manna L (2014) New materials for tunable plasmonic colloidal nanocrystals. Chem Soc Rev 43:3957–3975. doi:10.1039/c3cs60265f
Jain SC, McGregor JM, Roulston DJ (1990) Band-gap narrowing in novel III–V semiconductors. J Appl Phys 88:3747–3749
Zhao Y, Pan H, Lou Y et al (2009) Plasmonic Cu2−x S nanocrystals: optical and structural properties of copper-deficient copper(I) sulfides. J Am Chem Soc 131:4253–4261. doi:10.1021/ja805655b
Dorfs D, Härtling T, Miszta K et al (2011) Reversible tunability of the NIR valence band plasmon resonance in Cu2−x Se nanocrystals. J Am Chem Soc 133:11175–11180. doi:10.1021/ja2016284
Kriegel I, Jiang C, Rodríguez-Fernández J et al (2012) Tuning the excitonic and plasmonic properties of copper chalcogenide nanocrystals. J Am Chem Soc 134:1583–1590. doi:10.1021/ja207798q
Xie Y, Carbone L, Nobile C et al (2013) Metallic-like stoichiometric copper sulfide nanocrystals: phase- and shape-selective synthesis, near-infrared surface plasmon resonance properties, and their modeling. ACS Nano 7:7352–7369. doi:10.1021/nn403035s
West PR, Ishii S, Naik GV et al (2010) Searching for better plasmonic materials. Laser Photonics Rev 4:795–808. doi:10.1002/lpor.200900055
Teranishi T, Eguchi M, Kanehara M, Gwo S (2011) Controlled localized surface plasmon resonance wavelength for conductive nanoparticles over the ultraviolet to near-infrared region. J Mater Chem 21:10238. doi:10.1039/c0jm04545d
Rafea MA, Farag AAM, Roushdy N (2012) Controlling the crystallite size and influence of the film thickness on the optical and electrical characteristics of nanocrystalline Cu2S films. Mater Res Bull 47:257–266. doi:10.1016/j.materresbull.2011.11.035
Dhondge AD, Gosavi SR, Gosavi NM (2015) Influence of thickness on the photosensing properties of chemically synthesized copper sulfide thin films. World J Condens Matter Phys 5:1–9
Dhanam M, Manoj PK, Prabhu RR (2005) High-temperature conductivity in chemical bath deposited copper selenide thin films. J Cryst Growth 280:425–435. doi:10.1016/j.jcrysgro.2005.01.111
Franzen S (2008) Surface plasmon polaritons and screened plasma absorption in indium tin oxide compared to silver and gold. J Phys Chem C 112:6027–6032. doi:10.1021/jp7097813
Losego MD, Efremenko AY, Rhodes CL et al (2009) Conductive oxide thin films: model systems for understanding and controlling surface plasmon resonance. J Appl Phys 106:24903. doi:10.1063/1.3174440
Zang Z, Nakamura A, Temmyo J (2013) Single cuprous oxide films synthesized by radical oxidation at low temperature for PV application. Opt Express 21:11448. doi:10.1364/OE.21.011448
Zang Z, Nakamura A, Temmyo J (2013) Nitrogen doping in cuprous oxide films synthesized by radical oxidation at low temperature. Mater Lett 92:188–191. doi:10.1016/j.matlet.2012.10.083
Caballero R, Guillén C, Gutiérrez MT, Kaufmann CA (2006) CuIn1−x Ga x Se2-based thin-film solar cells by the selenization of sequentially evaporated metallic layers. Prog Photovolt Res Appl 14:145–153
López-García J, Placidi M, Fontané X et al (2015) CuIn1−x Al x Se2 thin film solar cells with depth gradient composition prepared by selenization of evaporated metallic precursors. Sol Energy Mater Sol Cells 132:245–251. doi:10.1016/j.solmat.2014.09.003
López-García J, Guillén C (2009) Adjustment of the selenium amount provided during formation of CuInSe2 thin films from the metallic precursors. Phys Status Solidi Appl Mater Sci 206:84–90
Xue MZ, Zhou YN, Zhang B et al (2006) Fabrication and electrochemical characterization of copper selenide thin films by pulsed laser deposition. J Electrochem Soc 153:A2262–A2268. doi:10.1149/1.2358854
Liu L, Liu C, Fu W et al (2016) Phase transformations of copper sulfide nanocrystals: towards highly efficient quantum-dot-sensitized solar cells. ChemPhysChem 17:771–776. doi:10.1002/cphc.201500627
Selivanov EN, Gulyaeva RI, Vershinin AD (2007) Thermal expansion and phase transformations of copper sulfides. Inorg Mater 43:573–578. doi:10.1134/S0020168507060027
Skomorokhov AN, Trots DM, Knapp M et al (2006) Structural behaviour of beta-Cu2−x Se (x = 0, 0.15, 0.25) in dependence on temperature studied by synchrotron powder diffraction. J Alloys Compd 421:64–71. doi:10.1016/j.jallcom.2005.10.079
Jackson AJ, Tiana D, Walsh A (2016) A universal chemical potential for sulfur vapours. Chem Sci 7:1082–1092. doi:10.1039/C5SC03088A
Olin A, Noläng B, Osadchii EG et al (2005) Chemical thermodynamics of selenium. Elsevier, Amsterdam
Gobeaut A, Laffont L, Tarascon JM et al (2009) Influence of secondary phases during annealing on re-crystallization of CuInSe2 electrodeposited films. Thin Solid Films 517:4436–4442. doi:10.1016/j.tsf.2009.01.043
Cuevas A, Romero R, Leinen D et al (2015) Effect of the stoichiometry of Cu x S thin films on the optical and electrical properties and the solar thermal performance. Sol Energy Mater Sol Cells 134:199–208. doi:10.1016/j.solmat.2014.11.048
Li J, Jiu TG, Tao GH et al (2014) Manipulating surface ligands of copper sulfide nanocrystals: synthesis, characterization, and application to organic solar cells. J Colloid Interface Sci 419:142–147. doi:10.1016/j.jcis.2013.12.057
Wu Y, Wadia C, Ma W et al (2008) Synthesis and photovoltaic application of copper(I) sulfide nanocrystals. Nano Lett 8:2551–2555. doi:10.1021/nl801817d
Luther JM, Jain PK, Ewers T, Alivisatos AP (2011) Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat Mater 10:361–366. doi:10.1038/nmat3004
Isac L, Popovici I, Enesca A, Duta A (2010) Copper sulfide (Cu x S) thin films as possible p-type absorbers in 3D solar cells. Energy Proc 2:71–78. doi:10.1016/j.egypro.2010.07.013
Sharma R, Sagade AA, Gosavi SR et al (2009) Effect of high electronic energy loss of 100 MeV gold heavy ions in copper chalcogenides (CuX, X = S, Se) at nanoscale: opto-electronic properties study. J Non Cryst Solids 355:1653–1658. doi:10.1016/j.jnoncrysol.2009.05.055
Viezbicke BD, Patel S, Davis BE, Birnie DP (2015) Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys Status Solidi 252:1700–1710. doi:10.1002/pssb.201552007
Barnabe A, Thimont Y, Lalanne M et al (2015) P-type conducting transparent characteristics of delafossite Mg-doped CuCrO2 thin films prepared by RF-sputtering. J Mater Chem C 3:6012–6024. doi:10.1039/C5TC01070E
Guillén C, Herrero J (2016) Structural and plasmonic characteristics of sputtered SnO2: Sb and ZnO: Al thin films as a function of their thickness. J Mater Sci 51:7276–7285. doi:10.1007/s10853-016-0010-9
Hsu SW, Ngo C, Tao AR (2014) Tunable and directional plasmonic coupling within semiconductor nanodisk assemblies. Nano Lett 14:2372–2380. doi:10.1021/nl404777h
Liu M, Xue X, Ghosh C et al (2015) Room-temperature synthesis of covellite nanoplatelets with broadly tunable localized surface plasmon resonance. Chem Mater 27:2584–2590. doi:10.1021/acs.chemmater.5b00270
Quintana-Ramirez PV, Arenas-Arrocena MC, Santos-Cruz J et al (2014) Growth evolution and phase transition from chalcocite to digenite in nanocrystalline copper sulfide: morphological, optical and electrical properties. Beilstein J Nanotechnol 5:1542–1552. doi:10.3762/bjnano.5.166
Grozdanov I, Najdoski M (1995) Optical and electrical properties of copper sulfide films of variable composition. J Solid State Chem 114:469–475. doi:10.1006/jssc.1995.1070
Rodríguez-Lazcano Y, Martínez H, Calixto-Rodríguez M, Núñez Rodríguez A (2009) Properties of CuS thin films treated in air plasma. Thin Solid Films 517:5951–5955. doi:10.1016/j.tsf.2009.03.075
Haram SK, Santhanam KSV (1994) Electroless deposition of orthorhombic copper (I) selenide and its room temperature phase transformation to cubic structure. Thin Solid Films 238:21–26. doi:10.1016/0040-6090(94)90642-4
Hu Y, Afzaal M, Malik MA, O’Brien P (2006) Deposition of copper selenide thin films and nanoparticles. J Cryst Growth 297:61–65. doi:10.1016/j.jcrysgro.2006.08.038
Bhuvaneswari PV, Ramamurthi K, Ramesh Babu R, Moorthy Babu S (2015) Structural, morphological, optical and electrical properties of Cu0.87Se thin films coated by electron beam evaporation method. Appl Phys A 120:1113–1120. doi:10.1007/s00339-015-9286-x
Lu JG, Fujita S, Kawaharamura T et al (2007) Carrier concentration dependence of band gap shift in n-type ZnO: Al films. J Appl Phys 101:83705. doi:10.1063/1.2721374
Kim WM, Kim JS, Jeong JH et al (2013) Analysis of optical band-gap shift in impurity doped ZnO thin films by using nonparabolic conduction band parameters. Thin Solid Films 531:430–435. doi:10.1016/j.tsf.2013.01.078
Kasap S, Koughia C, Ruda H, Johanson R (2006) Electrical conduction in metals and semiconductors. In: Kasap S (ed) Springer handbook of electronic and photonic materials. Springer, Boston, pp 19–45
Ellmer K (2001) Resistivity of polycrystalline zinc oxide films: current status and physical limit. J Phys D Appl Phys 34:3097–3108. doi:10.1088/0022-3727/34/21/301
Mendelsberg RJ, Garcia G, Li H et al (2012) Understanding the plasmon resonance in ensembles of degenerately doped semiconductor nanocrystals. J Phys Chem C 116:12226–12231. doi:10.1021/jp302732s
Ruda HE (1991) Compensation and transport characteristics of n-ZnTe. J Phys D Appl Phys 24:1158–1162. doi:10.1088/0022-3727/24/7/021
Acknowledgements
This work has been supported by the Spanish Ministry of Economy, Industry and Competitiveness through the MAT2015-66649-R project and the CIEMAT Photovoltaic Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guillén, C., Herrero, J. Nanocrystalline copper sulfide and copper selenide thin films with p-type metallic behavior. J Mater Sci 52, 13886–13896 (2017). https://doi.org/10.1007/s10853-017-1489-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1489-4