Abstract
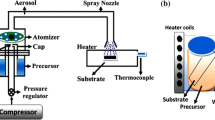
Zinc oxide (ZnO) nanorods of various morphologies are grown on zinc substrate by pressure-assisted hydrothermal process and the growth mechanism is investigated with the help of molecular dynamics (MD) simulation results. Hydrothermally reacted ZnO2 nanostructure bottom-up formation from Zn substrate is a useful process employed here. A systematic study on the role of process control parameters such as pressure and temperature on nanorod growth has been carried out. Correlation among the process parameters to form ordered nanostructures is established. The effect of pressure on the diameter and length of the grown ZnO nanorod structures is studied, which is precisely tunable. With a decrease in pressure from 500 to 400 kPa, the nanorod diameter is reduced by 22.2 %, while its length is increased by 24.8 %. At lower vapor pressure, the nanorod tips are sharper, whereas at higher vapor pressure they are flat. These variations along with a detailed analysis of MD simulations helps us hypothesize that pressure plays an important role in governing the diffusion of oxygen atom onto zinc surface and generating wurtzite phase. Simulation results clearly show that ZnO nanorods lift off due to their interaction with the Zn atoms on the substrate and the resulting forces.










Similar content being viewed by others
References
Huang MH, Mao S, Feick H, Yan H, Wu Y, Kind H, Weber E, Russo R, Yang P (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292:1897–1899
Keis K, Vayssieres L, Lindquist SE, Hagfeldt A (1999) Nanostructured ZnO electrodes for photovoltaic applications. Nanostruct Mater 12:487–490
Minne SC, Manalis SR, Quate CF (1995) Parallel atomic force microscopy using cantilevers with integrated piezoresistive sensors and integrated piezoelectric actuators. Appl Phys Lett 67:3918–3920
Shibata T, Unno K, Makino E, Ito Y, Shimada S (2002) Characterization of sputtered ZnO thin film as sensor and actuator for diamond AFM probe. Sens Actuators A 102:106–113
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Yan Y, Zhang Y, Meng G, Zhang L (2006) Synthesis of ZnO nanocrystals with novel hierarchical structures via atmosphere pressure physical vapor deposition method. J Cryst Growth 294:184–190
Peng X, Wickham J, Alivisatos AP (1998) Kinetics of II–VI and III–V colloidal semiconductor nanocrystal growth: focusing of size distributions. Am Chem Soc 120:5343–5344
Peng X (2003) Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv Mater 15:459–463
Zhao XQ, Kim CR, Lee JY, Heo JH, Shin CM, Ryu H, Chang JH, Lee HC, Son CS, Lee WJ, Jung WG, Tan ST, Zhao JL, Sun XW (2009) Effects of buffer layer annealing temperature on the structural and optical properties of hydrothermal growth ZnO. Appl Surf Sci 255:4461–4465
Pal E, Hornok V, Oszko A, Dekany I (2009) Hydrothermal synthesis of prism-like and flower-like ZnO and indium-doped ZnO structures. Colloid Surf A 340:1–9
Tong Y, Dong L, Liu Y, Zhao D, Zhang J, Lu Y, Shen D, Fan X (2007) Growth and optical properties of ZnO nanorods by introducing ZnO sols prior to hydrothermal process. Mater Lett 61:3578–3581
Corso AD, Posternak M, Resta R, Baldereschi A (1994) Ab initio study of piezoelectricity and spontaneous polarization in ZnO. Phys Rev B 50:10715–10721
Tiwary CS, Vishnu D, Kole AK, Brahmanandam J, Mahapatra DR, Kumbhakar P, Chattopadhyay K (2016) Stabilization of the high-temperature and high-pressure cubic phase of ZnO by temperature-controlled milling. J Mater Sci 51:126–137. doi:10.1007/s10853-015-9394-1
Zhao Y, Jiang YJ, Fang Y (2007) The influence of substrate temperature on ZnO thin films prepared by PLD technique. J Cryst Growth 307:278–282
Lim JM, Lee CM (2007) Effects of substrate temperature on the microstructure and photoluminescence properties of ZnO thin films prepared by atomic layer deposition. Thin Solid Films 515:3335–3338
Akshaya KB, Pritam D, Indrani T, Sriparna C, Shyamal C (2015) Temporal wetting property of ‘‘micro’’ versus ‘‘nano’’ rods of ZnO grown using the pressure dependent aqueous solution method. New J Chem 39:8993–8998
Yang J, Lang J, Yang L, Zhang Y, Wang D, Fan H, Liu H, Wang Y, Gao M (2008) Low-temperature growth and optical properties of ZnO nanorods. J Alloys Compd 450:521–524
Zhang J, Sun L, Liao C, Yan C (2002) A simple route towards tubular ZnO. Chem Commun 262(2002):262–263
Wang YW, Zhang LD, Wang GZ, Peng XS, Chu ZQ, Liang CH (2002) Catalytic growth of semiconducting zinc oxide nanowires and their photoluminescence properties. J Cryst Growth 234:171–175
Hu JQ, Li Q, Wong NB, Lee CS, Lee ST (2002) Synthesis of uniform hexagonal prismatic ZnO whiskers. Chem Mater 14:1216–1219
Badre C, Pauporte V, Turmine M, Dubot P, Lincot D (2008) Water-repellent ZnO nanowires films obtained by octadecylsilane self-assembled monolayers. Physica E 40:2454–2456
Yamamoto K, Nagasawa K, Ohmori T (2004) Preparation and characterization of ZnO nanowires. Physica E 24:129–132
Zafar HI, Kimleang K, Martin E, Mohammad A, Muhammad A, Anees A, Magnus W (2013) Hydrothermal growth of vertically aligned ZnO nanorods using a biocomposite seed layer of ZnO nanoparticles. Materials 6:3584–3597
Haili L, Shujie J, Shanshan B, Hongtao L, Shiyong G, Jinzhong W, Qingjiang Y, Fengyun G, Liancheng Z (2014) Precursor-controlled synthesis of different ZnO nanostructures by the hydrothermal method. Phys Status Solidi A 211:595–600
Liu W, Huang Q, Huang T, Cao P, Han S, Jia F, Zhu D, Ma X, Lul Y (2016) Secondary growth” in hydrothermal synthesis of aligned ZnO nanostructures and its application in dye-sensitized solar cells. J Nanosci Nanotechnol 16(4):4016–4022
Tong Y, Liu Y, Dong L, Zhao D, Zhang J, Lu Y, Shen D, Fan X (2006) Growth of ZnO nanostructures with different morphologies by using hydrothermal technique. J Phys Chem 110:20263–20267
Demyanets LN, Lyutin VI (2008) Status of hydrothermal growth of bulk ZnO: latest issues and advantages. J Cryst Growth 310:993–999
Dalal SH, Baptista DL, Teo KBK, Lacerda RG, Jefferson DA, Milne WI (2006) Controllable growth of vertically aligned zinc oxide nanowires using vapour deposition. Nanotechnology 17:4811–4818
Xu S, Wang ZL (2011) One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res 4:1013–1098
Henni A, Merrouche A, Telli L, Walter S, Azizi A, Fenineche N (2015) Effect of H2O2 concentration on electrochemical growth and properties of vertically oriented ZnO nanorods electrodeposited from chloride solutions. Mater Sci Semicond Process 40:585–590
Meyer B, Marx D (2003) Density-functional study of the structure and stability of ZnO surfaces. Phys Rev B 67:035403–035414
Wang ZL (2004) Zinc oxide nanostructures: growth, properties, and applications. J Phys 16:R829–R858
Shi L, Bao K, Cao J, Qian Y (2009) Growth and characterization of ZnS porous nanoribbon array constructed by connected nanocrystallites. Cryst Eng Comm 11:2308–2312
Saunders RB, McGlynn E, Henry MO (2011) Theoretical analysis of nucleation and growth of ZnO nanostructures in vapor phase transport growth. Cryst Growth Des 11:4581–4587
Liang T, Shan TR, Cheng YT, Devine BD, Noordhoek M, Li Y, Lu Z, Phillpot SR, Sinnott SB (2013) Classical atomistic simulations of surfaces and heterogeneous interfaces with the charge-optimized many body (COMB) potentials. Mater Sci Eng 74:255–279
Wang W, Pi Z, Lei F, Lu Y (2016) Understanding the tensile behaviors of ultra-thin ZnO nanowires via molecular dynamics simulations. AIP Adv 6:035111
Rappe AK, Goddard WA (1991) Charge equilibration for molecular dynamics simulations. J Phys Chem 95:3358–3363
Swope WC, Andersen HC, Berens PH, Wilson KR (1982) A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters. J Chem Phys 76:637–649
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31:1695–1697
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comp Phys 117:1–19
Acknowledgements
The authors acknowledge the financial support of Aeronautics R&D Board, Government of India, under the ACECOST phase-III program to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasireddi, R., Javvaji, B., Vardhan, H. et al. Growth of zinc oxide nanorod structures: pressure controlled hydrothermal process and growth mechanism. J Mater Sci 52, 2007–2020 (2017). https://doi.org/10.1007/s10853-016-0489-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0489-0