Abstract
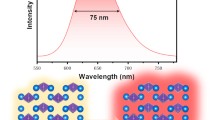
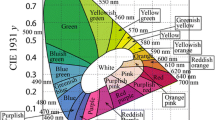
A series of scheelite-type Eu3+-activated CaMoO4 phosphors were synthesized by the nitrate–citrate gel combustion method. All the compounds crystallized in the tetragonal structure with space group I4 1 /a (No. 88). FESEM results reveal the spherical-like morphology. The CaMoO4 phosphor exhibited broad emission centered at 500 nm under the excitation of 298 nm wavelength, while Eu3+-activated CaMoO4 shows an intense characteristic red emission peak at 615 nm at different excitation wavelengths, due to 5D0 → 7F2 transition of Eu3+ ions. The intensities of transitions between different J levels depend on the symmetry of the local environment of Eu3+ ions and were estimated using the Judd–Ofelt analysis. The high asymmetric ratio revealed that Eu3+ occupies sites with a low symmetry and without an inversion center. The CIE chromaticity co-ordinates (x, y) were calculated from emission spectra, and the values were close to the NTSC standard. Therefore, the present phosphor is highly useful for LEDs applications.











Similar content being viewed by others
References
Sun L, Guo Q, Wu X, Luo S, Pan W, Huang K, Lu J, Ren L, Cao M, Hu C (2007) Synthesis and photoluminescent properties of strontium tungstate nanostructures. J Phys Chem C 111:532–537
Kroger FA (1948) Some aspect of the luminescence of solids. Elsevier, Amsterdam
Driscoll SA, Ozkan US (1994) Isotopic labeling studies on oxidative coupling of methane over alkali promoted molybdate catalysts. Stud Surf Sci Catal 82:367–375
Tanaka K, Miyajima T, Shirai N, Zhang Q, Nakata R (1995) Laser photochemical ablation of CdWO4 studied with the time-of-flight mass spectrometric technique. J Appl Phys 77:6581–6587
Phuruangrat A, Thongtem T, Thongtem S (2009) Preparation, characterization and photoluminescence of nanocrystalline calcium molybdates. J Alloy Compd 481:568–572
Graser R, Pitt E, Scharmann A, Zimmerer G (1975) Optical properties of CaWO4 and CaMoO4 crystals in the 4 to 25 eV region. Phys Status Solidi B 69:359–368
Yu P, Bi J, Xiao DQ, Chen LP, Jin XL, Yang ZN (2006) Preparation and microstructure of CaMoO4 ceramic films prepared through electrochemical technique. J Electroceram 16:473–476
Yoon JW, Ryu JH, Shim KB (2006) Photoluminescence in nanocrystalline MMoO4 (M = Ca, Ba) synthesized by a polymerized complex method. Mater Sci Eng B 127:154–158
Ryu JH, Choi BG, Yoon JW, Shim KB, Machi K, Hamada K (2007) Synthesis of CaMoO4 image nanoparticles by pulsed laser ablation in deionized water and optical properties. J Lumin 124:67–70
Thongtem T, Phuruangrat A, Thongtem S (2010) Microwave-assisted synthesis and characterization of SrMoO4 and SrWO4 nanocrystals. J Nanopart Res 12:2287–2294
Momma K, Izumi F (2008) VESTA: a three-dimensional visualization system for electronic and structural analysis. J Appl Crystallogr 41:653–658
Klug P, Alexander LE (1954) X-ray diffraction procedure. Wiley, New York
William GK, Hall WH (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall 1:22–31
Sczancoski JC, Bomio MDR, Cavalcante LS, Joya MR, Pizani PS, Varela JA, Longo E, Siu Li M, Andres JA (2009) Morphology and blue photoluminescence emission of PbMoO4 processed in conventional hydrothermal. J Phys Chem C 113:5812–5822
Tauc J (1970) Optical properties of solids. North-Holland, Amsterdam
Yoon JW, Choi CJ, Kim D (2011) Laser-induced synthesis of CaMoO4 nanocolloidal suspension and its optical properties. Mater Trans, JIM 52:768–771
Zhang F, Sfeir MY, Misewich JA, Wong SS (2008) Room-temperature preparation, characterization, and photoluminescence measurements of solid solutions of various compositionally-defined single-crystalline alkaline-earth-metal tungstate nanorods. Chem Mater 20:5500–5512
Polak K, Nikl M, Nitsch K, Kobayashi M, Ishii M, Usuki Y, Jarolimek O (1997) The blue luminescence of PbWO4 single crystals. J Lumin 72–74:781–783
Nikl M, Bohacek P, Mihokove E, Kobayashi M, Ishii M, Usuki Y, Babin V, Stolovich A, Zazubovich S, Bacci M (2000) Excitonic emission of scheelite tungstates AWO4 (A = Pb, Ca, Ba, Sr). J Lumin 87–89:1136–1139
Van Tol J, Van Der Waals JH (1996) The lowest triplet state of luminescent scheelites: a study of the MoO4 2− ion by electron paramagnetic resonance with optical detection. Mol Phys 88:803–820
Dieke GH (1968) Spectra and energy levels of rare-earth ions in crystals. Interscience Publishers, New York
Song HJ, Zhou LQ, Huang Y, Li L, Wang T, Yang L (2013) Synthesis, characterization and luminescent properties of La2Zr2O7:Eu3+ nanorods. Chin J Chem Phys 26:83–87
Jack K (2007) Video demystified: a handbook for the digital engineer, 5th edn. Newnes, New York
Ofelt GS (1962) Intensities of crystal spectra of rare-earth ions. J Chem Phys 37:511–519
Rakov N, Amaral DF, Guimaraes RB, Maciel GS (2010) Spectroscopic analysis of Eu3+ -and Eu3+:Yb3+-doped yttrium silicate crystalline powders prepared by combustion synthesis. J Appl Phys 108:073501–073506
Grzyb T, Lis S (2011) Structural and spectroscopic properties of LaOF: Eu3+ nanocrystals prepared by the sol–gel pechini method. Inorg Chem 50:8112–8120
Kumar V, Kumar V, Som S, Duvenhage MM, Ntwaeaborwa OM, Swart HC (2014) Effect of Eu doping on the photoluminescence properties of ZnO nanophosphors for red emission applications. Appl Surf Sci 308:419–430
Aruna ST, Mukasyan AS (2008) Combustion synthesis and nanomaterials. Curr Opin Solid State Mater Sci 12:44–50
McCamy CS (1992) Correlated color temperature as an explicit function of chromaticity coordinates. Color Res Appl 17:142–144
Chen BJ, Pun EYB, Lin H (2009) Photoluminescence and spectral parameters of Eu3+ in sodium–aluminum–tellurite ceramics. J Alloy Compd 479:352–356
Reisfeld R, Zigansky E, Gaft M (2004) Europium probe for estimation of site symmetry in glass films, glasses and crystals. Mol Phys 102:1319–1330
Cross AM, May PS, van Veggel FCJM, Berry MT (2010) Dipicolinate sensitization of europium luminescence in dispersible 5 % Eu: LaF3 nanoparticles. J Phys Chem C 114:14740–14747
Acknowledgements
One of the authors (R.S.) greatly acknowledges Professor Ashok K. Nagawat and Dr. K.V.R. Rao, Centre for Converging Technologies, University of Rajasthan for encouragement. Authors are thankful to DST-IRHPA for FESEM and EDX facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saraf, R., Shivakumara, C., Dhananjaya, N. et al. Photoluminescence properties of Eu3+-activated CaMoO4 phosphors for WLEDs applications and its Judd–Ofelt analysis. J Mater Sci 50, 287–298 (2015). https://doi.org/10.1007/s10853-014-8587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8587-3