Abstract
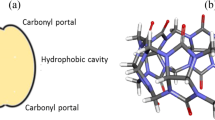
The complexation behavior of diprotonated phenylenediamine isomers with decamethylene cucurbit[6]uril (Me12Q[6]) in 1:1 and 1:2 stoichiometry was investigated in vacuum and solution using density functional theory (DFT). Among the isomers, o-phenylenediamine (oPDA) forms an exclusion complex in 1:1 stoichiometry, while others form an inclusion complex. In the 1:2 stoichiometry, at least one of the guest molecule lie on the surface of the Me12Q[6] cavity. The structural reorganization was found to depend on the mode of interaction of the guest molecule. In the gas phase, the enthalpy and Gibbs free energy are negative for all the complexes demonstrating the encapsulation process is spontaneous and thermodynamically favorable. In the solution phase, the enthalpy and entropy are negative for complexes with guest molecules meta- and para-isomers of phenylenediamine. For oPDA as a guest, the enthalpy and entropy are positive indicating the complex formation to be nonspontaneous. The MESP isosurface of complexes shows a higher accumulation of charge in the 1:2 stoichiometry, which reduces their stability. AIM analysis shows the interaction between oPDA and Me12Q[6] is due to hydrogen bonding with moderate strength, while for the other isomers, interactions between the benzene group and the Me12Q[6] cavity were noticed. EDA analysis shows the larger contribution is the electrostatic attraction and it decreases with the increase in the guest ratio. The formation of inclusion complexes by mPDA and pPDA and the surface adsorption of oPDA on Me12Q[6] and the higher accumulation of positive charge during solvation in oPDA@Me12Q[6] complexes make them labile in the solution phase.
Graphical abstract






Similar content being viewed by others
References
Freewan, W.A., Mock, W.L., Shih, N.-Y.: Cucurbituril. J. Am. Chem. Soc. 103, 7367–7368 (1981). https://doi.org/10.1021/ja00414a070
Lagona, J., Mukhopadhyay, P., Chakrabarti, S., Isaacs, L.: The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005). https://doi.org/10.1002/anie.200460675
Kim, J., Jung, I.-S., Kim, S.-Y., Lee, E., Kang, J.-K., Sakamoto, S., Yamaguchi, K., Kim, K.: New cucurbituril homologs: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n=5,7, and 8). J. Am. Chem. Soc. 122, 540–541 (2000). https://doi.org/10.1021/ja993376p
Isaacs, L., Park, S.-K., Liu, S., Ko, Y.H., Selvapalam, N., Kim, Y., Kim, H., Zavalij, P.Y., Ki, G.-H., Lee, H.-S., Kim, K.: The inverted cucurbit[n]uril family. J. Am. Chem. Soc. 127, 18000–18001 (2005). https://doi.org/10.1021/ja056988k
Kim, S.K., Park, K.M., Singha, K., Kim, J., Ahn, Y., Kim, K., Kim, W.J.: Galactosylated cucurbituril-inclusion polyplex for hepatocyte-targeted gene delivery. Chem. Commun. 46, 692–694 (2010). https://doi.org/10.1039/B920753H
Zhao, J., Kim, H.-J., Kim, S.-Y., Lee, J.W., Sakamoto, S., Yamaguchi, K., Kim, O.: Cucurbit[n]uril derivatives soluble in water and organic solvents. Angew. Chem. Int. Ed. 40, 4233–4235 (2001). https://doi.org/10.1002/1521-3773(20011119)40:22%3c4233::AID-ANIE4233%3e3.0.CO;2-D
Kim, K., Selvapalam, N., Oh, D.H.: Cucurbiturils—a new family of host molecules. J. Incl. Phenom. Macrocycl. Chem. 50, 31–36 (2004). https://doi.org/10.1007/s10847-004-8835-7
Ghosh, S.K., Dhamija, A., Ko, Y.H., An, J., Hur, M.Y., Boraste, D.R., Seo, J., Lee, E., Park, K.M., Kim, K.: Superacid-mediated functionalization of hydroxylated cucurbit[n]urils. J. Am. Chem. Soc. 141, 17503–17506 (2019). https://doi.org/10.1021/jacs.9b09639
Chubarova, E.V., Sokolov, M.N., Samsonenko, D.G., Vincent, C., Fedin, V.P.: Supramolecular compounds of chloroaquacomplexes {Mo3Q4(H2O)9−xClx}(4–x) + (Q = S, Se; x=2,3,5) with cucurbit[n]urils. J. Struct. Chem. 47, 939–945 (2006). https://doi.org/10.1007/s10947-006-0411-8
Fedin, V.P., Virovets, A.V., Sokolov, M.N., Dybtsev, D.N., Gerasko, O.A., Clegg, W.: Supramolecular assemblies based on cucurbituril adducts of hydrogen-bonded molybdenum and tungsten incomplete cuboidal aqua complexes. Inorg. Chem. 39, 2227–2230 (2000). https://doi.org/10.1021/ic000026r
Lin, R.-G., Long, L.-S., Huang, R.-B., Zheng, L.-S.: Directing role of hydrophobic–hydrophobic and hydrophilic–hydrophilic interactions in the self-assembly of calixarenes/cucurbiturils-based architectures. Cryst. Growth Des. 8, 791–794 (2008). https://doi.org/10.1021/cg701084x
Efremova, O.A., Mironov, Y.V., Kuratieva, N.V., Fedorov, V.E.: Novel supramolecular compounds based on cucurbit[6]uril, 1,8-diaminooctane and octahedral thiohydroxo anions with cluster core {Re6S8}. Inorg. Chem. Acta 363, 4411–4415 (2010). https://doi.org/10.1016/j.ica.2010.06.032
Tian, X., Chen, L.X., Yao, Y.Q., Chen, K., Chen, M.-D., Zhu, X., Tao, Z.: 4-Sulfocali[4]arene/cucurbit[7]uril-based supramolecular assemblies through the outer surface interactions of cucurbit[n]uril. ACS Omega 3, 6665–6672 (2018). https://doi.org/10.1021/acsomega.8b00829
Ni, X.-L., Xiao, X., Cong, H., Zhu, Q.-J., Xue, S.-F., Tao, Z.: Self-assemblies based on the “outer-surface interactions” of cucurbit[n]urils: new opportunities for supramolecular architectures and materials. Acc. Chem. Res. 47, 1386–1395 (2014). https://doi.org/10.1021/ar5000133
Ji, N.-N., Cheng, X.-J., Zhao, Y., Liang, L.-L., Ni, X.-N., Xiao, X., Zhu, Q.-J., Xue, S.-F., Dong, N., Tao, Z.: Tetrachloridometallate dianion-incuded cucurbit[8]uril supramolecular assemblies with large channels and their potential applications for extraction coating on solid-phase microextraction fibres. Inorg. Chem. 53, 21–23 (2014). https://doi.org/10.1021/ic4025684
Smitha, B., Suhanya, D., Sridhar, S., Ramakrishna, M.: Separation of organic–organic mixtures by pervaporation—a review. J. Membr. Sci. 241, 1–21 (2004). https://doi.org/10.1016/j.memsci.2004.03.042
Kandambeth, S., Mallick, A., Lukose, B., Mane, M.V., Heine, T., Banerjee, R.: Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19254–19527 (2012). https://doi.org/10.1021/ja308278w
Lin, K., Gόmez-Bombarelli, R., Beh, E.S., Tong, L., Chen, Q., Valle, A., Aspuru-Guzik, A., Aziz, M.J., Gordon, R.G.: A redox-flow battery with an alloxazine-based organic electrolyte. Nat. Energy 1, 16102 (2016). https://doi.org/10.1038/nenergy.2016.102
Friedemann, M.N., Robinson, S.W., Gerhardt, G.A.: o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal. Chem. 68, 2621–2628 (1996). https://doi.org/10.1021/ac960093w
Sagasser, J., Ma, B.N., Baecker, D., Salcher, S., Hermann, M., Lamprecht, J., Angerer, S., Obexer, P., Kircher, B., Gust, R.: A new approach in cancer treatment: discovery of chloro[N,N-disalicydine-1,2-phenylenediamine]iron(III) complexes a ferroptosis inducers. J. Med. Chem. 62, 8053–8061 (2019). https://doi.org/10.1021/acs.jmedchem.9b00814
Hajiaghababaei, L., Eslambolipour, M., Badiei, A., Ganjali, M.R., Ziarani, G.M.: Controlled release of anticancer drug using o-phenylenediamine functionalized SBA-15 as a novel nanocarrier. Chem. Pap. 75, 1841–1850 (2021). https://doi.org/10.1007/s11696-020-01422-9
Alaqeel, S.I.: Synthetic approaches to benzimidazoles from o-phenylenediamine: a literature review. J. Saudi Chem. Soc. 21, 229–237 (2017). https://doi.org/10.1016/j.jscs.2016.08.001
Li, B., Xu, K., Wang, Y., Su, H., Chi, L., Li, C.: Selective complexation and efficient separation of cis/trans-1,2-dichloroethene isomers by a pillar[5]arene. RSC Adv. 10, 45112–45115 (2020). https://doi.org/10.1039/D0RA09307F
Shan, P., Lin, R., Liu, M., Tao, Z., Xiao, X., Liu, J.: Recognition of glycine by cucurbit[5]uril and cucurbit[6]uril: a comparative study of exo- and endo-binding. Chin. Chem. Lett. 32, 2301–2304 (2021). https://doi.org/10.1016/j.cclet.2021.02.020
Liu, L., Nouvel, N., Scherman, O.A.: Controlled catch and release of small molecules with cucurbit[6]uril via a kinetic trap. Chem. Commun. (2009). https://doi.org/10.1039/B903033F
Hirani, Z., Taylor, H.F., Babcock, E.F., Bockus, A.T., Varnado, C.D., Bielawski, C.W., Urbach, A.R.: Molecular recognition of methionine-terminated peptides by cucucrbit[8]uril. J. Am. Chem. Soc. 140, 12263–12269 (2018). https://doi.org/10.1021/jacs.8b07865
Cao, L., Zhang, Y.-Q., Lin, R.-L., Liu, J.-X., Tao, Z.: Controllable synthesis of dodecamethylcucurbit[6]uril and its application in separating phenylenediamine isomers. Cryst. Growth Des. 21, 2993–2999 (2021). https://doi.org/10.1021/acs.cgd.1c00141
Cao, L., Guo, H.-L., Lin, R.-L., Zhang, Z.-H., Tian, L.-F., Liu, J.-X., Tao, Z.: Separation of phenylenediamine isomers by using decamethylcucurbit[5]uril. New J. Chem. 45, 2754–2759 (2021). https://doi.org/10.1039/D0NJ05999D
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B.G., Gomperts, R., Mennucci, B., Hratchian, H.P., Ortiz, J.V., Izmaylov, A.F., Sonnenberg, J.L., Williams-Young, D., Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V.G., Gao, J., Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery Jr, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J.J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Keith, T.A., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Millam, J.M., Klene, M., Adamo, C., Cammi, R., Ochterski, J.W., Martin, R.L., Morokuma, K., Farkas, O., Foresman, J.B., Fox, D.J.: Gaussian, Inc., Wallingford CT (2016)
Grimme, S.: Supramolecular binding thermodynamics by disperstion-corrected density functional theory. Chem. Eur. J. 18, 9955–9964 (2012). https://doi.org/10.1002/chem.201200497
Klamt, A., Jonas, V., Bürger, T., Lohrenz, J.C.W.: Refinement and parameterization of COSMO-RS. J. Phys. Chem. A 102, 5074–5085 (1998). https://doi.org/10.1021/jp980017s
AIMAll (Version 19.10.12), Todd A. Keith, TK Gristmill Software, Overland Park KS (aim.tkgristmill.com) (2019)
Venkataramanan, N.S., Suvitha, A.: Encapsulation of sulfur, oxygen and nitrogen mustards by cucurbiturils: a DFT study. J. Incl. Phenom. Macrocycl. Chem. 83, 387–400 (2015). https://doi.org/10.1007/s10847-015-0575-y
Lu, T., Chen, F.W.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). https://doi.org/10.1002/jcc.22885
Velde, G.T., Bickelhaupt, F.M., Baerends, E.J., Fonseca Guerra, C., van Gisbergen, S.J.A., Snijders, J.G., Ziegler, T.: Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001). https://doi.org/10.1002/jcc.1056
Venkataramanan, N.S., Suvitha, A., Sahara, R., Kawazoe, Y.: A computational study on the complexation of bisbenzimdazolyl derivatives with cucurbituril and cyclohexylcucurbituril. J. Incl. Phenom. Macrocycl. Chem. 100, 217–231 (2021). https://doi.org/10.1007/s10847-021-01078-2
Mecozzi, S., Rebek, J., Jr.: The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J. 4, 1016–1022 (1998). https://doi.org/10.1002/(SICI)1521-3765(19980615)4:6%3c1016::AID-CHEM1016%3e3.0.CO;2-B
Suvitha, A., Venkataramanan, N.S.: Trapping of organophosphorus chemical nerve agents by pillar[5]arene: A DFT, AIM, NCI and EDA analysis. J. Incl. Phenom. Macrocycl. Chem. 87, 207-218 (2017). https://doi.org/10.1007/s10847-017-0691-y
Yoosefian, M., Mola, A.: Solvent effects on binding energy, stability order and hydrogen bonding of guanine-cytosine base pair. J. Mol. Liq. 209, 526–530 (2015). https://doi.org/10.1016/j.molliq.2015.06.029
Suh, S.B., Kim, J.C., Choi, Y.C., Yun, S., Kim, K.S.: Nature of one-dimensional short hydrogen bonding: bond distances, bond energies, and solvent effects. J. Am. Chem. Soc. 126, 2186–2193 (2004). https://doi.org/10.1021/ja037607a
Brzezinski, B., Zundel, G.: Influence of solvents on intramolecular hydrogen bonds with large proton polarizability. J. Magn. Reson. 48, 361–366 (1982). https://doi.org/10.1016/0022-2364(82)90070-1
Sin, K.-R., Ko, S.-G., Kim, C.-J., Pak, S.-H., Kim, H.-C., Kim, C.-U.: Quantum chemical investigation on interaction of 5-fluorouracil with cucurbiturils. Montash Chem. 151, 721–727 (2020). https://doi.org/10.1007/s00706-020-02599-1
Ma, F., Zheng, X., Xie, J., Li, Z.: Binding properties of cucurbit[7]uril to neutral and protonated amino acids: a theoretical study. Int. J. Quantum Chem. 121, e26491 (2020). https://doi.org/10.1002/qua.26491
Cheriet, M., Madi, F., Nouar, L., Lafifi, I., Himri, S., Merabet, N., Khatmi, D.: A DFT study of inclusion complexes of the antituberculosis drugs pyrazinamide and isoniazid with cucurbit[7]uril. J. Incl. Phenom. Macrocycl. Chem. 89, 127–136 (2017). https://doi.org/10.1007/s10847-017-0738-0
Venkataramanan, N.S., Suvitha, A., Kawazoe, Y.: Unraveling the binding nature of hexane with quinone functionalized pillar[5]quinone: a computational study. J. Incl. Phenom. Macrocycl. Chem. 95, 307–319 (2019). https://doi.org/10.1007/s10847-019-00945-3
Bidermann, F., Nau, W.M., Schneider, H.-J.: The hydrophobic effect revisited-studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew. Chem. Int. Ed. 53, 11158 (2014). https://doi.org/10.1002/anie.201310958
Bidermann, F., Vendruscolo, M., Scherman, O.A., De Simone, A., Nau, W.M.: Cucurbit[8]uril and blue-box: high-energy water release overwhelms electrostatic interactions. J. Am. Chem. Soc. 135, 14879 (2013). https://doi.org/10.1021/ja407951x
Zhou, F., Wang, J., Yuping, Z., Wang, Q., Guo, C., Wang, F., Zhang, H.: Comparative studies on the effect of CB[8] on the charge transfer interaction. Theor. Chem. Acc. 138, 50 (2019). https://doi.org/10.1007/s00214-019-2447-9
Li, L., Jia, R., Zheng, Q.-C.: What are the effects of cucurbit[n]urils on CTMS loading? Insights from QM calculations and MD simulations. Comput. Mater. Sci. 181, 109751 (2020). https://doi.org/10.1016/j.commatsci.2020.109751
Hassanzadeh, K., Akhtari, K., Esmaeili, S.S., Vaziri, A., Zamani, H., Maghsoodi, M., Noori, S., Moradi, A., Hamidi, P.: Encapsulation of thiotepa and altretamine as neurotoxic anticancer drugs in cucurbit[n]uril (n=7,8) nanocapsules: a DFT study. J. Theor. Comput. Chem. 15, 1650056 (2016). https://doi.org/10.1142/S0219633616500565
Bulat, F.A., Toro-Labbe, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16, 1679–1691 (2010). https://doi.org/10.1007/s00894-010-0692-x
Venkataramanan, N.S., Suvitha, A., Kawazoe, Y.: Density functional theory study on the dihydrogen bond cooperativity in the growth behavior of dimethyl sulfoxide clusters. J. Mol. Liq. 249, 452–462 (2018). https://doi.org/10.1016/j.molliq.2017.11.062
Koch, U., Popelier, P.L.A.: Characterization of C–H–O hydrogen bonds on the basis of the charge density. J. Phys. Chem. A 99, 9747–9754 (1995). https://doi.org/10.1021/j100024a016
Yahioui, K., Seridi, L., Mansouri, K.: Temozolomide binding to cucurbit[7]uril: QTAIM, NCI-RDG and NBO analyses. J. Incl. Phenom. Macrocycl. Chem. 99, 61–77 (2021). https://doi.org/10.1007/s10847-020-01027-5
Rozas, I., Alkorta, I., Elguero, J.: Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J. Am. Chem. Soc. 122, 11154–11161 (2000). https://doi.org/10.1021/ja0017864
Ulloa, C.O., Ponce-Vargas, M., Munoz-Castro, A.: Nature of cucurbituril-halogen encapsulation. Structural and interaction energy consideration in the X2@CB[n] (X=Cl, Br, I, n=6, 7, 8) from relativistic DFT calculations. Phys. Chem. Chem. Phys. 20, 29325 (2018). https://doi.org/10.1039/C8CP04936J
Reany, O., Li, A., Yefet, M., Gilson, M.K., Keinan, E.: Attractive interactions between heteroallenes and cucurbituril portal. J. Am. Chem. Soc. 139, 8138–8145 (2017). https://doi.org/10.1021/jacs.6b13005
Acknowledgements
The authors would like to thank the staff of the Center for Computational Materials Science, Institute for Materials Research, Tohoku University, and the supercomputer resource through the HPCI System Research Project (Project ID: hphp200040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Natarajan Sathiyamoorthy, V., Suvitha, A. & Sahara, R. Molecular insights into the complex formation between dodecamethylcucurbit[6]uril and phenylenediamine isomers. J Incl Phenom Macrocycl Chem 102, 637–651 (2022). https://doi.org/10.1007/s10847-022-01144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01144-3