Abstract
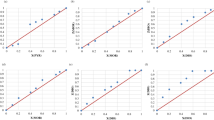
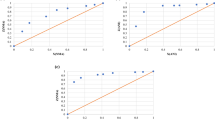
trans-N,N′-Bis(9-phenyl-9-xanthenyl)cyclohexane-1,2-diamine (1,2-DAX) and trans-N,N′-bis(9-phenyl-9-thioxanthenyl)cyclohexane-1,2-diamine (1,2-DAT) were recrystallized independently from each of the xylene isomers (o-Xy, m-Xy and p-Xy) and ethylbenzene (EB) to ascertain their host ability for these guest solvents. It was thus shown that both compounds were capable of forming complexes with each guest species. The hosts were then recrystallized from various mixtures of these solvents in order to establish if they possessed any preference for a particular guest species from the series, with the view to possibly offering an alternative separation strategy for such mixtures using host‒guest chemistry principles and 1,2-DAX and/or 1,2-DAT. In so doing, each host compound was revealed to have comparable selectivities, favouring o-Xy and discriminating against p-Xy. This contrasted markedly with earlier findings reported for related host compounds 1,4-DAX and 1,4-DAT, which both preferred p-Xy whilst displaying a bias against o-Xy. We subsequently calculated the void volumes that remained after removal of each of the guest species from the packing calculation and found that the preferred guests always occupied the smallest volumes compared with guests that were less favoured (for complexes of 1,2-DAX, 1,2-DAT, 1,4-DAX and 1,4-DAT). This finding implied that the favoured guest species, here, experienced a tighter packing than any of the other solvents from the series. Additional to this information, data from single crystal diffraction and thermal analyses for each complex are also provided here.





Similar content being viewed by others
References
Lusi, M., Barbour, L.J.: Solid-vapour sorption of xylenes: prioritized selectivity as a means of separating all three isomers using a single substrate. Angew. Chem. Int. Ed. 51, 3928–3931 (2012)
Fabri, J., Graeser, U., Simo, T.A.: “Xylenes". Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim (2000)
Meyer, M.: “Xylidines”. Ullmann’s Encylclopedia of Industrial Chemistry. Wiley-VCH, Weinheim (2012)
Fadzil, N.A.M., Rahim, M.H.A., Maniam, G.P.: A brief review of para-xylene oxidation to terephthalic acid as a model of primary C-H bond activation. Chin. J. Catal. 35, 1641–1652 (2014)
Sholl, D.S., Lively, R.P.: Seven chemical separations to change the world. Nature 532, 435–437 (2016)
Welch, V.A., Fallon, K.J., Gelbke, H.-P.: “Ethylbenzene” Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim (2005)
Santos, K.A.O., Dantas Neto, A.A., Moura, M.C.P.A., Castro Dantas, T.N.: Separation of xylene isomers through adsorption on microporous materials: a review. Braz. J. Pet. Gas 5, 255–268 (2011)
Cannella, W.J.: Kirk-Othmer Encyclopedia of Chemical Technology. (2007). https://doi.org/10.1002/0471238961.2425120503011414.a01.pub2
Wicht, M.M., Bathori, N.B., Nassimbeni, L.R.: Isoquinoline-based Werner clathrates with xylene isomers: aromatic interactions vs. molecular flexibility. Dalton Trans. 44, 6863–6870 (2015)
Alaerts, L., Kirschhock, C.E.A., Maes, M., van der Veen, M.A., Finsy, V., Depla, A., Martens, J.A., Baron, G.V., Jacobs, P.A., Denayer, J.F.M., de Vos, D.E.: Selective adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem. Int. Ed. 46, 4293–4297 (2007)
Vermoortele, F., Maes, M., Moghadam, P.Z., Lennox, M.J., Ragon, F., Boulhout, M., Biswas, S., Laurier, K.G.M., Beurroies, I., Denoyel, R., Roeffaers, M., Stock, N., Düren, T., Serre, C., De Vos, D.E.: p-Xylene-selective metal–organic frameworks: a case of topology-directed selectivity. J. Am. Chem. Soc. 133, 18526–18529 (2011)
Devi, R.N., Edgar, M., Gonzalez, J., Slawin, A.M.Z., Tunstall, D.P., Grewal, P., Cox, P.A., Wright, P.A.: Structural studies and computer simulation of the inclusion of aromatic hydrocarbons in a zinc 2,6-naphthalene dicarboxylate framework compound. J. Phys. Chem. B 108, 535–543 (2004)
Mukherjee, S., Joarder, B., Manna, B., Desai, A.V., Chaudhari, A.K., Ghosh, S.K.: Framework-flexibility driven selective sorption of p-xylene over other isomers by a dynamic metal-organic framework. Sci. Rep. 4, 5761 (2015)
Warren, J.E., Perkins, C.G., Jelfs, K.E., Boldrin, P., Chater, P.A., Miller, G.J., Manning, T.D., Briggs, M.E., Stylianou, K.C., Claridge, J.B., Rosseinsky, M.J.: Shape selectivity by guest-drivenrRestructuring of a porous material. Angew. Chem. Int. Ed. 53, 4592–4596 (2014)
Nassimbeni, L.R., Bathori, N.B., Patel, L.D., Su, H., Weber, E.: Separation of xylenes by enclathration. Chem. Commun. 51, 3627–3629 (2015)
Toda, F., Tanaka, K., Wang, Y., Lee, G.-H.: Inclusion complexes of 1,1,2,2-tetraphenylethane-1,2-diol and 1,1,2,2-tetraphenylethane. Chem. Lett. 15, 109–112 (1986)
Barton, B., Hosten, E.C., Pohl, P.L.: Discrimination between o-xylene, m-xylene, p-xylene and ethylbenzene by host compound (R,R)-(−)-2,3-dimethoxy-1,1,4,4-tetraphenylbutane-1,4-diol. Tetrahedron 72, 8099–8105 (2016)
Barton, B., Caira, M.R., de Jager, L., Hosten, E.C.: N, N′-Bis(9-phenyl-9-thioxanthenyl)ethylenediamine: highly selective host behavior in the presence of xylene and ethylbenzene guest mixtures. Cryst. Growth Des. 17, 6660–6667 (2017)
Barton, B., Jager, L., Hosten, E.C.: Minor modifications afford improved host selectivities in xanthenyl-type host systems. CrystEngComm 21, 3000–3013 (2019)
Barton, B., Senekal, U., Hosten, E.C.: Compounds N,N′-bis(9-cycloheXy-9-xanthenyl)ethylenediamine and its thio derivative, N,N′-bis(9-cycloheXy-9-thioxanthenyl)ethylenediamine, as potential hosts in the presence of xylenes and ethylbenzene: conformational analyses and molecular modelling considerations. Tetrahedron 75, 3399–3412 (2019)
Barton, B., Jooste, D.V., Hosten, E.C.: Synthesis and assessment of compounds trans-N,N′-bis(9-phenyl-9-xanthenyl)cyclohexane-1,4-diamine and trans-N,N′-bis(9-phenyl-9-thioxanthenyl)cyclohexane-1,4-diamine as hosts for potential xylene and ethylbenzene guests. J. Incl. Phenom. Macrocycl. Chem. 93, 333–346 (2019)
Bruker, A.X.S.: APEX2, SADABS and SAINT. Bruker, A.X.S., Madison (2010)
Sheldrick, G.M.: SHELXT-integrated space-group and crystal structure determination. Acta Crystallogr. A 71, 3–8 (2015)
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015)
Hübschle, C.B., Sheldrick, G.M., Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011)
Macrae, C.F., Sovago, I., Cottrell, S.J., Galek, P.T.A., McCabe, P., Pidcock, E., Platings, M., Shields, G.P., Stevens, J.S., Towler, M., Wood, P.A.: Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Cryst. 53, 226–235 (2020)
Barton, B., Jooste, D.V., Hosten, E.C.: trans-N, N′-Bis(9-phenyl-9-xanthenyl)cyclohexane-1,2-diamine and its thioxanthenyl derivative as potential host compounds for the separation of anilines through host-guest chemistry principles. J. Incl. Phenom. Macrocycl. Chem. 97, 159–174 (2020)
Sykes, N.M., Su, H., Weber, E., Bourne, S.A., Nassimbeni, L.R.: Selective enclathration of methyl- and dimethylpiperidines by fluorenol hosts. Cryst. Growth Des. 17, 819–826 (2017)
Barton, B., McCleland, C.W., Caira, M.R., de Jager, L., Hosten, E.C.: Crystal X-ray diffraction and molecular modelling considerations elucidate the factors responsible for the opposing host behaviour of two isostructural xanthenyl- and thioxanthenyl-derived host compounds. Cryst. Growth Des. 19, 2396–2418 (2019)
Acknowledgements
Financial support is acknowledged from the Nelson Mandela University and the National Research Foundation (NRF). Prof Mino Caira (UCT) is thanked for fruitful discussions involving crystals voids and volumes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barton, B., Jooste, D.V. & Hosten, E.C. Behaviour of host compounds 1,2-DAX and 1,2-DAT in the presence of mixed xylene and ethylbenzene guest solvents, and comparisons with their 1,4 host derivatives. J Incl Phenom Macrocycl Chem 100, 155–167 (2021). https://doi.org/10.1007/s10847-021-01065-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01065-7