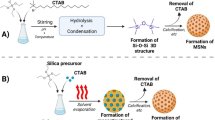
Abstract
Although various therapies have been developed for cancer treatment, chemotherapy plays a vital role, but still faces many challenges, such as severe cytotoxicity, side effects, multidrug resistance, and poor tumor selectivity. The development of targeted drug delivery has provided new strategies for addressing the limitations of the conventional chemotherapy, and has become more significant in clinical research in recent times. Among the various stimuli, pH triggered delivery is regarded as the most general strategy, targeting the acidic extracellular microenvironment and intracellular organelles of solid tumors. It is well-known that the extracellular pH of most tumor tissues is more acidic (pH 6.5–6.8) than that of normal tissues (pH 7.4). In our present review, we focus on some of the recent literature reports on the fabrication and application of pH-sensitive smart nanoparticles for tumor targeted drug delivery system. The strategies to the chemical design of these nanocarriers and their clinical findings are discussed. Particular focus is given to silica, chitosan, and silica–chitosan based nanocarriers. These smart nanoparticles will have a promising platform in improving human health and quality of life.












Similar content being viewed by others
References
Shpaisman, N., Sheihet, L., Bushman, J., Winters, J., Kohn, J.: One-step synthesis of biodegradable curcumin-derived hydrogels as potential soft tissue fillers after breast cancer surgery. Biomacromolecules 13(8), 2279–2286 (2012). doi:10.1021/bm300518e
Guan, T., Shang, W., Li, H., Yang, X., Fang, C., Tian, J., Wang, K.: From detection to resection: photoacoustic tomography and surgery guidance with indocyanine green loaded gold Nanorod@liposome core-shell nanoparticles in liver cancer. Bioconjug Chem. 28(4), 1221–1228 (2017). doi:10.1021/acs.bioconjchem.7b00065
Ma, N., Jiang, Y.W., Zhang, X., Wu, H., Myers, J.N., Liu, P., Jin, H., Gu, N., He, N., Wu, F.G., Chen, Z.: Enhanced radiosensitization of gold nanospikes via hyperthermia in combined cancer radiation and photothermal therapy. ACS Appl. Mater. Interfaces (2016). doi:10.1021/acsami.6b10132
Detappe, A., Thomas, E., Tibbitt, M.W., Kunjachan, S., Zavidij, O., Parnandi, N., Reznichenko, E., Lux, F., Tillement, O., Berbeco, R.: Ultrasmall silica-based bismuth gadolinium nanoparticles for dual magnetic resonance-computed tomography image guided radiation therapy. Nano Lett. 17(3), 1733–1740 (2017). doi:10.1021/acs.nanolett.6b05055
Cai, X., Luo, Y., Yan, H., Du, D., Lin, Y.: pH-responsive ZnO nanocluster for lung cancer chemotherapy. ACS Appl. Mater. Interfaces 9(7), 5739–5747 (2017). doi:10.1021/acsami.6b13776
Seib, F.P., Tsurkan, M., Freudenberg, U., Kaplan, D.L., Werner, C.: Heparin-modified polyethylene glycol microparticle aggregates for focal cancer chemotherapy. ACS Biomater. Sci. Eng. 2(12), 2287–2293 (2016). doi:10.1021/acsbiomaterials.6b00495
Giroux Leprieur, E., Dumenil, C., Julie, C., Giraud, V., Dumoulin, J., Labrune, S., Chinet, T.: Immunotherapy revolutionises non-small-cell lung cancer therapy: results, perspectives and new challenges. Eur. J. Cancer 78, 16–23 (2017). doi:10.1016/j.ejca.2016.12.041
Tsai, H.F., Hsu, P.N.: Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J. Biomed. Sci. 24(1), 35 (2017). doi:10.1186/s12929-017-0341-0
Cheng, J., Tan, G., Li, W., Zhang, H., Wu, X., Wang, Z., Jin, Y.: Facile synthesis of chitosan assisted multifunctional magnetic Fe3O4@SiO2@CS@pyropheophorbide-a fluorescent nanoparticles for photodynamic therapy. New J. Chem. 40(10), 8522–8534 (2016). doi:10.1039/c6nj01765g
Choi, S.Y., Baek, S.H., Chang, S.J., Song, Y., Rafique, R., Lee, K.T., Park, T.J.: Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosens. Bioelectron. 93, 267–273 (2017). doi:10.1016/j.bios.2016.08.094
Zheng, M., Li, Y., Liu, S., Wang, W., Xie, Z., Jing, X.: One-pot to synthesize multifunctional carbon dots for near infrared fluorescence imaging and photothermal cancer therapy. ACS Appl. Mater. Interfaces 8(36), 23533–23541 (2016). doi:10.1021/acsami.6b07453
Zhou, B., Li, Y., Niu, G., Lan, M., Jia, Q., Liang, Q.: Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl. Mater. Interfaces 8(44), 29899–29905 (2016). doi:10.1021/acsami.6b07838
Shi, P., Qu, K., Wang, J., Li, M., Ren, J., Qu, X.: pH-responsive NIR enhanced drug release from gold nanocages possesses high potency against cancer cells. Chem. Commun. 48(61), 7640–7642 (2012). doi:10.1039/c2cc33543c
Meng, Z., Chen, X., Liu, Z., Chen, S., Yu, N., Wei, P., Chen, Z., Zhu, M.: NIR-laser-triggered smart full-polymer nanogels for synergic photothermal-/chemo-therapy of tumors. RSC Adv. 6(93), 90111–90119 (2016). doi:10.1039/c6ra20432e
Yang, H., Xu, M., Li, S., Shen, X., Li, T., Yan, J., Zhang, C., Wu, C., Zeng, H., Liu, Y.: Chitosan hybrid nanoparticles as a theranostic platform for targeted doxorubicin/VEGF shRNA co-delivery and dual-modality fluorescence imaging. RSC Adv. 6(35), 29685–29696 (2016). doi:10.1039/c6ra03843c
Cui, N., Zhu, S.-H.: Monoclonal antibody-tagged polyethylenimine (PEI)/poly(lactide) (PLA) nanoparticles for the enhanced delivery of doxorubicin in HER-positive breast cancers. RSC Adv. 6(83), 79822–79829 (2016). doi:10.1039/c6ra12616b
Zhao, W., Wei, J.S., Zhang, P., Chen, J., Kong, J.L., Sun, L.H., Xiong, H.M., Mohwald, H.: Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells. ACS Appl. Mater. Interfaces 9(22), 18474–18481 (2017). doi:10.1021/acsami.7b02542
Li, D., Ma, Y., Du, J., Tao, W., Du, X., Yang, X., Wang, J.: Tumor acidity/NIR controlled interaction of transformable nanoparticle with biological systems for cancer therapy. Nano Lett. 17(5), 2871–2878 (2017). doi:10.1021/acs.nanolett.6b05396
Yang, Z., Tian, R., Wu, J., Fan, Q., Yung, B.C., Niu, G., Jacobson, O., Wang, Z., Liu, G., Yu, G., Huang, W., Song, J., Chen, X.: Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano 11(4), 4247–4255 (2017). doi:10.1021/acsnano.7b01261
Li, Y., Hu, H., Zhou, Q., Ao, Y., Xiao, C., Wan, J., Wan, Y., Xu, H., Li, Z., Yang, X.: Alpha-amylase- and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 9(22), 19215–19230 (2017). doi:10.1021/acsami.7b04066
Makwana, H., Mastrotto, F., Magnusson, J.P., Sleep, D., Hay, J., Nicholls, K.J., Allen, S., Alexander, C.: Engineered polymer-transferrin conjugates as self-assembling targeted drug delivery systems. Biomacromolecules. 18(5), 1532–1543 (2017). doi:10.1021/acs.biomac.7b00101
Ge, J., Zhang, Y., Dong, Z., Jia, J., Zhu, J., Miao, X., Yan, B.: Initiation of targeted nanodrug delivery in vivo by a multifunctional magnetic implant. ACS Appl. Mater. Interfaces (2017). doi:10.1021/acsami.7b05009
Yap, T.A., Carden, C.P., Kaye, S.B.: Beyond chemotherapy: targeted therapies in ovarian cancer. Nat. Rev. Cancer 9(3), 167–181 (2009). doi:10.1038/nrc2583
Kim, D.K., Dobson, J.: Nanomedicine for targeted drug delivery. Yearb. Pediatr. 19, 6294–6307 (2009)
Jeon, S.J., Hauser, A.W., Hayward, R.C.: Shape-morphing materials from stimuli-responsive hydrogel hybrids. Acc. Chem. Res. 50(2), 161–169 (2017). doi:10.1021/acs.accounts.6b00570
Abbaszad Rafi, A., Mahkam, M., Davaran, S., Hamishehkar, H.: A smart pH-responsive nano-carrier as a drug delivery system: a hybrid system comprised of mesoporous nanosilica MCM-41 (as a nano-container) & a pH-sensitive polymer (as smart reversible gatekeepers): preparation, characterization and in vitro release studies of an anti-cancer drug. Eur. J. Pharm. Sci. 93, 64–73 (2016). doi:10.1016/j.ejps.2016.08.005
Kim, H., Kang, Y.J., Jeong, E.S., Kang, S., Kim, K.T.: Glucose-responsive disassembly of polymersomes of sequence-specific boroxole-containing block copolymers under physiologically relevant conditions. ACS Macro Lett. 1(10), 1194–1198 (2012). doi:10.1021/mz3004192
Amstad, E., Kim, S.H., Weitz, D.A.: Photo- and thermoresponsive polymersomes for triggered release. Angew. Chem. 51(50), 12499–12503 (2012). doi:10.1002/anie.201206531
Zhang, C.Y., Yang, Y.Q., Huang, T.X., Zhao, B., Guo, X.D., Wang, J.F., Zhang, L.J.: Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials 33(26), 6273–6283 (2012). doi:10.1016/j.biomaterials.2012.05.025
Feng, F., Li, R., Zhang, Q., Wang, Y., Yang, X., Duan, H., Yang, X.: Preparation of reduction-triggered degradable microcapsules for intracellular delivery of anti-cancer drug and gene. Polymer. 55(1), 110–118 (2014). doi:10.1016/j.polymer.2013.11.035
Lee, J.-E., Ahn, E., Bak, J.M., Jung, S.-H., Park, J.M., Kim, B.-S., Lee, H.-i.: Polymeric micelles based on photocleavable linkers tethered with a model drug. Polymer 55(6), 1436–1442 (2014). doi:10.1016/j.polymer.2014.01.026
Klinger, D., Landfester, K.: Stimuli-responsive microgels for the loading and release of functional compounds: fundamental concepts and applications. Polymer 53(23), 5209–5231 (2012). doi:10.1016/j.polymer.2012.08.053
Zheng, Q., Hao, Y., Ye, P., Guo, L., Wu, H., Guo, Q., Jiang, J., Fu, F., Chen, G.: A pH-responsive controlled release system using layered double hydroxide (LDH)-capped mesoporous silica nanoparticles. J. Mater. Chem. B 1(11), 1644 (2013). doi:10.1039/c3tb00518f
Schmaljohann, D.: Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 58(15), 1655–1670 (2006). doi:10.1016/j.addr.2006.09.020
Levine, D.J., Runcevski, T., Kapelewski, M.T., Keitz, B.K., Oktawiec, J., Reed, D.A., Mason, J.A., Jiang, H.Z., Colwell, K.A., Legendre, C.M., FitzGerald, S.A., Long, J.R.: Olsalazine-based metal-organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. J. Am. Chem. Soc. 138(32), 10143–10150 (2016). doi:10.1021/jacs.6b03523
Li, Y., Li, N., Pan, W., Yu, Z., Yang, L., Tang, B.: Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mater. Interfaces 9(3), 2123–2129 (2017). doi:10.1021/acsami.6b13876
Zeng, J., Du, P., Liu, L., Li, J., Tian, K., Jia, X., Zhao, X., Liu, P.: Superparamagnetic reduction/pH/temperature multistimuli-responsive nanoparticles for targeted and controlled antitumor drug delivery. Mol. Pharm. 12(12), 4188–4199 (2015). doi:10.1021/acs.molpharmaceut.5b00342
Liu, M., Gan, L., Chen, L., Xu, Z., Zhu, D., Hao, Z., Chen, L.: Supramolecular core-shell nanosilica@liposome nanocapsules for drug delivery. Langmuir 28(29), 10725–10732 (2012). doi:10.1021/la3021645
Yao, Y.Y., Gedda, G., Girma, W.M., Yen, C.L., Ling, Y.C., Chang, J.Y.: Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl. Mater. Interfaces 9(16), 13887–13899 (2017). doi:10.1021/acsami.7b01599
Yanagisawa, T.S.T., Kuroda, K., Kato, C.: Trimethylsilyl derivatives of alkyltrimethylammonium-kanemite complexes and their conversion to microporous silica materials. Bull. Chem. Soc. Jpn. 63(5), 1535–1537 (1990)
Tang, F., Li, L., Chen, D.: Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 24(12), 1504–1534 (2012). doi:10.1002/adma.201104763
Ying, W.D.Z.: On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 107(7), 2821–2860 (2007)
Alfredsson, V., Anderson, M.W.: Structure of MCM-48 revealed by transmission electron microscopy. Chem. Mater. 8(5), 1141–1146 (1996). doi:10.1021/cm950568k
Dongyuan, Z.J.F., Qisheng, H., Nicholas, M.G.H.F., Bradley, F.C., Galen, D.S.: Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores. Science 279, 548–552 (1998)
Xu, X., Lü, S., Gao, C., Feng, C., Wu, C., Bai, X., Gao, N., Wang, Z., Liu, M.: Self-fluorescent and stimuli-responsive mesoporous silica nanoparticles using a double-role curcumin gatekeeper for drug delivery. Chem. Eng. J. 300, 185–192 (2016). doi:10.1016/j.cej.2016.04.087
Chen, Y., Zhang, H., Cai, X., Ji, J., He, S., Zhai, G.: Multifunctional mesoporous silica nanocarriers for stimuli-responsive target delivery of anticancer drugs. RSC Adv. 6(94), 92073–92091 (2016). doi:10.1039/c6ra18062k
Slowing, I.I., Vivero-Escoto, J.L., Trewyn, B.G., Lin, V.S.Y.: Mesoporous silica nanoparticles: structural design and applications. J. Mater. Chem. 20(37), 7924 (2010). doi:10.1039/c0jm00554a
Chen, T., Wu, W., Xiao, H., Chen, Y., Chen, M., Li, J.: Intelligent drug delivery system based on mesoporous silica nanoparticles coated with an ultra-pH-sensitive gatekeeper and poly(ethylene glycol). ACS Macro Lett. 5(1), 55–58 (2016). doi:10.1021/acsmacrolett.5b00765
Yang, D., Yang, G., Gai, S., He, F., Lv, R., Dai, Y., Yang, P.: Imaging-guided and light-triggered chemo-/photodynamic/photothermal therapy based on Gd(III) chelated mesoporous silica hybrid spheres. ACS Biomater. Sci. Eng. 2(11), 2058–2071 (2016). doi:10.1021/acsbiomaterials.6b00462
Yi, Z., Hussain, H.I., Feng, C., Sun, D., She, F., Rookes, J.E., Cahill, D.M., Kong, L.: Functionalized mesoporous silica nanoparticles with redox-responsive short-chain gatekeepers for agrochemical delivery. ACS Appl. Mater. Interfaces 7(18), 9937–9946 (2015). doi:10.1021/acsami.5b02131
Baeza, A., Guisasola, E., Ruiz-Hernández, E., Vallet-Regí, M.: Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 24(3), 517–524 (2012). doi:10.1021/cm203000u
Cheng, Y.J., Luo, G.F., Zhu, J.Y., Xu, X.D., Zeng, X., Cheng, D.B., Li, Y.M., Wu, Y., Zhang, X.Z., Zhuo, R.X., He, F.: Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 7(17), 9078–9087 (2015). doi:10.1021/acsami.5b00752
Muhammad, F., Guo, M., Qi, W., Sun, F., Wang, A., Guo, Y., Zhu, G.: pH-triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J. Am. Chem. Soc. 133(23), 8778–8781 (2011). doi:10.1021/ja200328s
Zhang, J., Wu, D., Li, M.F., Feng, J.: Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable zno quantum dots for intracellular drug delivery. ACS Appl. Mater. Interfaces 7(48), 26666–26673 (2015). doi:10.1021/acsami.5b08460
Xue, M., Zhong, X., Shaposhnik, Z., Qu, Y., Tamanoi, F., Duan, X., Zink, J.I.: pH-operated mechanized porous silicon nanoparticles. J. Am. Chem. Soc. 133(23), 8798–8801 (2011). doi:10.1021/ja201252e
He, Y., Su, Z., Xue, L., Xu, H., Zhang, C.: Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J. Control. Release 229, 80–92 (2016). doi:10.1016/j.jconrel.2016.03.001
Roszak, A.W., McKendrick, K., Gardiner, A.T., Mitchell, I.A., Isaacs, N.W., Cogdell, R.J., Hashimoto, H., Frank, H.A.: Protein regulation of carotenoid binding; gatekeeper and locking amino acid residues in reaction centers of Rhodobacter sphaeroides. Structure. 12(5), 765–773 (2004). doi:10.1016/j.str.2004.02.037
Palanikumar, L., Choi, E.S., Cheon, J.Y., Joo, S.H., Ryu, J.-H.: Noncovalent polymer-gatekeeper in mesoporous silica nanoparticles as a targeted drug delivery platform. Adv. Funct. Mater. 25(6), 957–965 (2015). doi:10.1002/adfm.201402755
Han, U., Seo, Y., Hong, J.: Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 6, 24158 (2016). doi:10.1038/srep24158
Zhang, M., Liu, J., Kuang, Y., Li, Q., Zheng, D.W., Song, Q., Chen, H., Chen, X., Xu, Y., Li, C., Jiang, B.: Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int. J. Biol. Macromol. 98, 691–700 (2017). doi:10.1016/j.ijbiomac.2017.01.136
Qiu, L., Zhao, Y., Li, B., Wang, Z., Cao, L., Sun, L.: Triple-stimuli (protease/redox/pH) sensitive porous silica nanocarriers for drug delivery. Sens. Actuators B 240, 1066–1074 (2017). doi:10.1016/j.snb.2016.09.083
Huang, X., Hauptmann, N., Appelhans, D., Formanek, P., Frank, S., Kaskel, S., Temme, A., Voit, B.: Synthesis of hetero-polymer functionalized nanocarriers by combining surface-initiated ATRP and RAFT polymerization. Small. 8(23), 3579–3583 (2012). doi:10.1002/smll.201201397
Mei, X., Chen, D., Li, N., Xu, Q., Ge, J., Li, H., Lu, J.: Hollow mesoporous silica nanoparticles conjugated with pH-sensitive amphiphilic diblock polymer for controlled drug release. Microporous Mesoporous Mater. 152, 16–24 (2012). doi:10.1016/j.micromeso.2011.12.015
Yuan, L., Tang, Q., Yang, D., Zhang, J.Z., Zhang, F., Hu, J.: Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J. Phys. Chem. C. 115(20), 9926–9932 (2011). doi:10.1021/jp201053d
Zhang, Y., Ang, C.Y., Li, M., Tan, S.Y., Qu, Q., Luo, Z., Zhao, Y.: Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl. Mater. Interfaces 7(32), 18179–18187 (2015). doi:10.1021/acsami.5b05893
Chen, F., Zhu, Y.: Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: sensitive response to the narrow pH range. Mesoporous Mesoporous Mater. 150, 83–89 (2012). doi:10.1016/j.micromeso.2011.07.023
Chen, X., Liu, Z.: Dual responsive mesoporous silica nanoparticles for targeted co-delivery of hydrophobic and hydrophilic anticancer drugs to tumor cells. J. Mater. Chem. B. 4(25), 4382–4388 (2016). doi:10.1039/c6tb00694a
Li, G., Song, S., Guo, L., Ma, S.: Self-assembly of thermo- and pH-responsive poly(acrylic acid)-b-poly(N-isopropylacrylamide) micelles for drug delivery. J. Polym. Sci. A 46(15), 5028–5035 (2008). doi:10.1002/pola.22831
Xue, Y.-N., Huang, Z.-Z., Zhang, J.-T., Liu, M., Zhang, M., Huang, S.-W., Zhuo, R.-X.: Synthesis and self-assembly of amphiphilic poly(acrylic acid-b-dl-lactide) to form micelles for pH-responsive drug delivery. Polymer. 50(15), 3706–3713 (2009). doi:10.1016/j.polymer.2009.05.033
Pourjavadi, A., Tehrani, Z.M.: Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): a pH-sensitive carrier for gemcitabine delivery. Mater. Sci. Eng. C 61, 782–790 (2016). doi:10.1016/j.msec.2015.12.096
Chertok, B., Moffat, B.A., David, A.E., Yu, F., Bergemann, C., Ross, B.D., Yang, V.C.: Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 29(4), 487–496 (2008). doi:10.1016/j.biomaterials.2007.08.050
Rahimi, M., Safa, K.D., Alizadeh, E., Salehi, R.: Dendritic chitosan as a magnetic and biocompatible nanocarrier for the simultaneous delivery of doxorubicin and methotrexate to MCF-7 cell line. New J. Chem. 41(8), 3177–3189 (2017). doi:10.1039/c6nj04107h
Pourjavadi, A., Tehrani, Z.M., Shakerpoor, A.: Dendrimer-like supramolecular nanovalves based on polypseudorotaxane and mesoporous silica-coated magnetic graphene oxide: a potential pH-sensitive anticancer drug carrier. Supramol. Chem. 28(7–8), 624–633 (2016). doi:10.1080/10610278.2015.1089357
He, D., He, X., Wang, K., Zou, Z., Yang, X., Li, X.: Remote-controlled drug release from graphene oxide-capped mesoporous silica to cancer cells by photoinduced pH-jump activation. Langmuir 30(24), 7182–7189 (2014). doi:10.1021/la501075c
Wang, Y., Wang, K., Zhao, J., Liu, X., Bu, J., Yan, X., Huang, R.: Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 135(12), 4799–4804 (2013). doi:10.1021/ja312221g
Wang, T.T., Lan, J., Zhang, Y., Wu, Z.L., Li, C.M., Wang, J., Huang, C.Z.: Reduced graphene oxide gated mesoporous silica nanoparticles as a versatile chemo-photothermal therapy system through pH controllable release. J. Mater. Chem. B 3(30), 6377–6384 (2015). doi:10.1039/c5tb00824g
Kumar, M.N.V.R.: A review of chitin and chitosan applications. React. Funct. Polym. 46, 1–27 (2000)
Dash, M., Chiellini, F., Ottenbrite, R.M., Chiellini, E.: Chitosan: a versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 36(8), 981–1014 (2011). doi:10.1016/j.progpolymsci.2011.02.001
Guibal, E.: Heterogeneous catalysis on chitosan-based materials: a review. Prog. Polym. Sci. 30(1), 71–109 (2005). doi:10.1016/j.progpolymsci.2004.12.001
Ye, Y.Q., Yang, F.L., Hu, F.Q., Du, Y.Z., Yuan, H., Yu, H.Y.: Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin. Int. J. Pharm. 352(1–2), 294–301 (2008). doi:10.1016/j.ijpharm.2007.10.035
Peng, S.F., Yang, M.J., Su, C.J., Chen, H.L., Lee, P.W., Wei, M.C., Sung, H.W.: Effects of incorporation of poly(gamma-glutamic acid) in chitosan/DNA complex nanoparticles on cellular uptake and transfection efficiency. Biomaterials 30(9), 1797–1808 (2009). doi:10.1016/j.biomaterials.2008.12.019
Guo, Y., Chu, M., Tan, S., Zhao, S., Liu, H., Otieno, B.O., Yang, X., Xu, C., Zhang, Z.: Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol. Pharm. 11(1), 59–70 (2014). doi:10.1021/mp400514t
Nogueira-Librelotto, D.R., Scheeren, L.E., Vinardell, M.P., Mitjans, M., Rolim, C.M.: Chitosan-tripolyphosphate nanoparticles functionalized with a pH-responsive amphiphile improved the in vitro antineoplastic effects of doxorubicin. Colloids Surf. B 147, 326–335 (2016). doi:10.1016/j.colsurfb.2016.08.014
Prabha, G., Raj, V.: Preparation and characterization of polymer nanocomposites coated magnetic nanoparticles for drug delivery applications. J. Magnet. Magnet. Mater. 408, 26–34 (2016). doi:10.1016/j.jmmm.2016.01.070
Wu, W., Wu, Z., Yu, T., Jiang, C., Kim, W.S.: Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 16(2), 023501 (2015). doi:10.1088/1468-6996/16/2/023501
Ramezani, S., Ghazitabar, A., Sadrnezhaad, S.K.: Synthesis and characterization of chitosan coating of NiFe2O4 nanoparticles for biomedical applications. J. Iran. Chem. Soc. 13(11), 2069–2076 (2016). doi:10.1007/s13738-016-0924-9
Lu, A.H., Salabas, E.L., Schuth, F.: Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. 46(8), 1222–1244 (2007). doi:10.1002/anie.200602866
Scheeren, L.E., Nogueira, D.R., Macedo, L.B., Vinardell, M.P., Mitjans, M., Infante, M.R., Rolim, C.M.: PEGylated and poloxamer-modified chitosan nanoparticles incorporating a lysine-based surfactant for pH-triggered doxorubicin release. Colloids Surf. B 138, 117–127 (2016). doi:10.1016/j.colsurfb.2015.11.049
Parveen, S., Sahoo, S.K.: Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur. J. Pharmacol. 670(2–3), 372–383 (2011). doi:10.1016/j.ejphar.2011.09.023
Wang, J.J., Zeng, Z.W., Xiao, R.Z., Xie, T., Zhou, G.L., Zhan, X.R., Wang, S.L.: Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 6, 765–774 (2011). doi:10.2147/IJN.S17296
Kariminia, S., Shamsipur, A., Shamsipur, M.: Analytical characteristics and application of novel chitosan coated magnetic nanoparticles as an efficient drug delivery system for ciprofloxacin. Enhanced drug release kinetics by low-frequency ultrasounds. J. Pharm. Biomed. Anal. 129, 450–457 (2016). doi:10.1016/j.jpba.2016.07.016
Ding, Y., Yin, H., Shen, S., Sun, K., Liu, F.: Chitosan-based magnetic/fluorescent nanocomposites for cell labelling and controlled drug release. New J. Chem. 41(4), 1736–1743 (2017). doi:10.1039/c6nj02897g
Wang, F.Q., Li, P., Zhang, J.P., Wang, A.Q., Wei, Q.: A novel pH-sensitive magnetic alginate-chitosan beads for albendazole delivery. Drug Dev. Ind. Pharm. 36(7), 867–877 (2010). doi:10.3109/03639040903567117
Pourjavadi, A., Mazaheri Tehrani, Z., Jokar, S.: Chitosan based supramolecular polypseudorotaxane as a pH-responsive polymer and their hybridization with mesoporous silica-coated magnetic graphene oxide for triggered anticancer drug delivery. Polymer 76, 52–61 (2015). doi:10.1016/j.polymer.2015.08.050
Nogueira, D.R., Scheeren, L.E., Pilar Vinardell, M., Mitjans, M., Rosa Infante, M., Rolim, C.M.: Nanoparticles incorporating pH-responsive surfactants as a viable approach to improve the intracellular drug delivery. Mater. Sci. Eng. C 57, 100–106 (2015). doi:10.1016/j.msec.2015.07.036
Jin, Y.H., Hu, H.Y., Qiao, M.X., Zhu, J., Qi, J.W., Hu, C.J., Zhang, Q., Chen, D.W.: pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: preparation and in vitro evaluation. Colloids Surf. B 94, 184–191 (2012). doi:10.1016/j.colsurfb.2012.01.032
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deveci, P., Taner, B. & Albayatı, S.H.M. Mesoporous silica and chitosan based pH-sensitive smart nanoparticles for tumor targeted drug delivery. J Incl Phenom Macrocycl Chem 89, 15–27 (2017). https://doi.org/10.1007/s10847-017-0741-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0741-5