Abstract
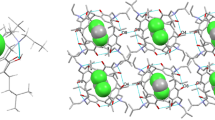
The synthesis of a C 4 dissymmetric resorcinarene tetracarboxylic acid derivative and determination of its critical micelle concentration is reported. The tetrahydroxy derivative was prepared by reduction of the tetra-acid. The low-temperature single crystal X-ray structure of the methyl ester derivative of the tetra-acid is also reported. This crystallised with two independent molecules of similar boat (flattened cone) conformation within the asymmetric unit.




Similar content being viewed by others
References
Nimse, S.B., Kim, T.: Biological applications of functionalized calixarenes. Chem. Soc. Rev. 42, 366–386 (2013)
Li, N., Harrison, R.G., Lamb, J.D.: Application of resorcinarene derivatives in chemical separations. J. Incl. Phenom. Macrocycl. Chem. 78, 39–60 (2014)
Kim, H.J., Lee, M.H., Mutihac, L., Vicens, J., Kim, J.S.: Host–guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 41, 1173–1190 (2012)
Kim, J.S., Quang, D.T.: Calixarene-derived fluorescent probes. Chem. Rev. 107, 3780–3799 (2007)
McIldowie, M.J., Mocerino, M., Ogden, M.I.: A brief review of C n -symmetric calixarenes and resorcinarenes. Supramol. Chem. 22, 13–39 (2010)
Szumna, A.: Inherently chiral concave molecules—from synthesis to applications. Chem. Soc. Rev. 39, 4274–4285 (2010)
Michels, J.J., Huskens, J., Engbersen, J.F.J., Reinhoudt, D.N.: Probing the interactions of calix[4]arene-based amphiphiles and cyclodextrins in water. Langmuir 16, 4864–4870 (2000)
Arimori, S., Nagasaki, T., Shinkai, S.: Self-assembly of tetracationic amphiphiles bearing a calix[4]arene core. Correlation between the core structure and the aggregation properties. J. Chem. Soc. Perkin Trans 2, 679–683 (1995)
Balasubramanian, R., Kim, B., Tripp, S.L., Wang, X., Lieberman, M., Wei, A.: Dispersion and stability studies of resorcinarene-encapsulated gold nanoparticles. Langmuir 18, 3676–3681 (2002)
Stavens, K.B., Pusztay, S.V., Zou, S., Andres, R.P., Wei, A.: Encapsulation of neutral gold nanoclusters by resorcinarenes. Langmuir 15, 8337–8339 (1999)
Rizzi, A.: Fundamental aspects of chiral separations by capillary electrophoresis. Electrophoresis 22, 3079–3106 (2001)
Amini, A.: Recent developments in chiral capillary electrophoresis and applications of this technique to pharmaceutical and biomedical analysis. Electrophoresis 22, 3107–3130 (2001)
Davidson, T.A., Mondal, K., Yang, X.: Use of a chiral surfactant for enantioselective reduction of a ketone. J. Colloid Interface Sci. 276, 498–502 (2004)
Baczko, K., Larpent, C., Lesot, P.: New amino acid-based anionic surfactants and their use as enantiodiscriminating lyotropic liquid crystalline NMR solvents. Tetrahedron Asymm. 15, 971–982 (2004)
Bazzanella, A., Mörbel, H., Bächmann, K., Milbradt, R., Böhmer, V., Vogt, W.: Highly efficient separation of amines by electrokinetic chromatography using resorcarene-octacarboxylic acids as pseudostationary phases. J. Chromatogr. A792, 143–149 (1997)
Wang, W., Zhu, X., Yan, C.: Determination of safranine T in food samples by CTAB sensitised fluorescence quenching method of the derivatives of calix[4]arene. Food Chem. 141, 2207–2212 (2013)
Ungaro, R., Pochini, A., Andreeti, G.D.: New ionisable ligands from p.t-butylcalix[4]arene. J. Incl. Phenom. 2, 199–206 (1984)
McKervey, M.A., Seward, E.M.: Molecular receptors. Synthesis and X-ray crystal structure of a calix[4]arene tetracarbonate-acetonitrile (1:1) clathrate. J. Org. Chem. 51, 3581–3584 (1986)
Buckley, B.R., Boxhall, J.Y., Page, P.C.B., Chan, Y., Elsegood, M.R.J., Heaney, H., Holmes, K.E., McIldowie, M.J., McKee, V., McGrath, M.J., Mocerino, M., Poulton, A.M., Sampler, E.P., Skelton, B.W., White, A.H.: Mannich and O-alkylation reactions of tetraalkocyresorcin[4]arenes—the use of some products in ligand-assisted reactions. Eur. J. Org. Chem. 2006, 5117-5134 (2006)
McIldowie, M.J., Mocerino, M., Skelton, B.W., White, A.H.: Facile Lewis acid catalyzed synthesis of C 4 symmetric resorcinarenes. Org. Lett. 2, 3869–3871 (2000)
Spencer, W., Sutter, J.R.: Kinetic study of the monomer-dimer equilibrium of methylene blue in aqueous solution. J. Phys. Chem. 83, 1573–1576 (1979)
Domínguez, A., Fernández, A., González, N., Iglesias, E., Montenegro, L.: Determination of critical micelle concentration of some surfactants by three techniques. J. Chem. Educ. 74, 1227–1231 (1997)
Rosen, M.J.: Surfactants and Interfacial Phenomena. Wiley, New York (1978)
Naemura, K., Miyabe, H., Shingai, Y., Tobe, Y.: Preparation and enantiomer recognition behaviour of crown ethers containing cis-1-phenylcyclohexane-1,2-diol and trans-1,2-diphenylcyclohexane-1,2-diol as a chiral subunit. J. Chem. Soc. Perkin Trans. 1, 1073–1077 (1993)
Pietraszkiewicz, M., Kozbial, M.: Enantiomeric differentiation of amino acids by a chiral crown ether derived from d-mannose studied by the liquid membrane technique. J. Incl. Phenom. Macrocycl. Chem. 14, 339–348 (1992)
Hyun, M.H., Han, S.C., Lipshutz, B.H., Shin, Y.-J., Welch, C.J.: Liquid chromatographic resolution of racemic amines, amino alcohols and related compounds on a chiral crown ether stationary phase. J. Chromatogr. A959, 75–83 (2002)
Favre, H.A., Hellwinkel, D., Powell, W.H., Smith Jr, H.A., Tsay, S.S.-C.: Phane nomenclature. Part II. Modification of the degree of hydrogenation and substitution derivatives of phane parent hydrides (IUPAC Recommendations 2002). Pure Appl. Chem. 74, 809–834 (2002)
Sheldrick, G.M.: A short history of SHELX. Acta Crystallogr. Sect. A 64, 112–122 (2008)
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Jack Harrowfield on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
McIldowie, M.J., Mocerino, M., Ogden, M.I. et al. C 4 Dissymmetric resorcinarene derivatives: synthesis, crystal structure and micelle formation. J Incl Phenom Macrocycl Chem 82, 47–51 (2015). https://doi.org/10.1007/s10847-015-0525-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0525-8