Abstract
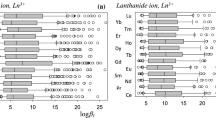
The increase use of ion sensors in different fields leads to many intensive studies to introduce sensing materials or selectophores in the fabrication of ion-selective electrodes. In this study the complexation stability of samarium ion with different ionophores was efficiently predicted by the QSPR model. Since the selectivity of ionophores can be defined by the stability constants of samarium–ionophore complexes, quantitative structure–property relationship (QSPR) studies on complex stability constants (log K) were carried out. The suitable subset of molecular descriptors was calculated and the genetic algorithm was employed to select those descriptors that resulted in the best-fit models. The multiple linear regression (MLR) method was utilized to construct the linear QSPR models. The best model showed most accurate predictions with the Leave-One-Out cross validation (Q 2LOO = 0.912), Leave-Group-Out cross validation (Q 2LGO = 0.907), external test set and Y-randomization. Also, the applicability domain of the model was analysed by the leverage approach. According to the best of our knowledge, this is the first research on QSPR studies for testing and estimating selectophores in samarium sensors based on complex stability constants. This report could be an experimental guide to find and design of highly selective sensors for Sm(III) ions.


Similar content being viewed by others
References
Ganjali, M.R., Rezapour, M.: Encyclopedia of Sensors, Potentiometric Ion Sensors, vol. 8, pp. 197–288. American Scientific Publisher (ASP), Stevenson Ranch (2006)
Fricker, S.P.: The therapeutic application of lanthanides. Chem. Soc. Rev. 35, 524–533 (2006)
Toropov, A.A., Toropova, A.P., Rasulev, B.F., Benfenati, E., Gini, G., Leszczynska, D., Leszczynski, J.: Coral, QSPR modeling of rate constants of reactions between organic aromatic pollutants and hydroxyl radical. J. Comput. Chem. 33, 1902–1906 (2012)
Li, D., Liu, M.S., Ji, B., Hwang, K.C., Yongga, H.: Identifying the molecular mechanics and binding dynamics characteristics of potent inhibitors to HIV-1 protease. Chem. Biol. Drug Des. 80, 440–454 (2012)
Fayet, G., Rotureau, P.A., Joubert, L., Adamo, C.: Development of a QSPR model for predicting thermal stabilities of nitroaromatic compounds taking into account their decomposition mechanisms. J. Mol. Model. 17, 2443–2453 (2011)
Goodarzi, M., Chen, T., Freitas, M.P.: QSPR predictions of heat of fusion of organic compounds using Bayesian regularized artificial neural networks. Chemom. Intell. Lab. Syst. 104, 260–264 (2010)
Katritzky, A.R., Stoyanova-Slavova, I.B., Dobchev, D.A., Karelson, M.: QSPR modeling of flash points: an update. J. Mol. Gr. Model. 26, 529–536 (2007)
Ahmadi, S.: Application of GA-MLR method in QSPR modeling of stability constants of diverse 15-crown-5 complexes with sodium cation. J. Incl. Phenom. Macrocycl. Chem. 74, 57–66 (2012)
Mirkhani, S.A., Gharagheizi, F., Farahani, N., Tumba, K.: Prediction of surface tension of ionic liquids by molecular approach. J. Mol. Liq. 179, 78–87 (2013)
Silla, J.M., Nunes, C.A., Cormanich, R.A., Guerreiro, M.C., Ramalho, T.C., Freitas, M.P.: MIA- QSPR and effect of variable selection on the modeling of kinetic parameters related to activities of modified peptides against dengue type 2. Chemom. Intell. Lab. Syst. 108, 146–149 (2011)
Kiani-Anbouhi, R., Ganjali, M.R., Norouzi, P.: Prediction of the complexation stabilities of La3+ ion with ionophores applied in lanthanoid sensors. J. Incl. Phenom. Macrocycl. Chem. 78, 325–336 (2014)
Kirk, O.R., Othmer, F.D.: Encyclopedia of Chemical Technology, vol. 19, p. 851. Wiley, New York (1982)
Fine, L.W., Beall, H.: Chemistry for Engineers and Scientists. Saunders, Philadelphia (1990)
Upadhyay, A., Singh, A.K., Jain, A.K., Gupta, V.K., Bandi, K.R.: Potentiometric study of coated graphite electrode and polymeric membrane electrode for the determination of Sm3+ ion. Electroanalysis 24, 1630–1638 (2012)
Rezaei, B., Askarpour, N., Hadadzadeh, H.: Experimental and semiempirical investigation of interaction between fast Sm membrane sensor and 1, 3-di(thiophene imino) benzoic acid. IEEE Sens. J. 11, 2077–2083 (2011)
Agrahari, S.K., Srivastava, A.K.: Graphite electrode coated with a 7,16-dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-multiwalled carbon nanotube composite as sensor for detection of samarium. Electroanalysis 23, 1531–1535 (2011)
Naddaf, E., Zamani, H.A.: Samarium(III)-PVC-membrane sensor based on ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline. Anal. Lett. 42, 2838–2852 (2009)
Zamani, H.A., Imani, A., Arvinfar, A., Rahimi, F., Ganjali, M.R., Faridbod, F., Meghdadi, S.: Neodymium(III)–PVC membrane sensor based on a new four dentate ionophore. Mater. Sci. Eng. C 31, 588–592 (2011)
Faridbod, F., Ganjali, M.R., Larijani, B., Hosseini, M., Norouzi, P.: Ho3+ carbon paste sensor based on multi-walled carbon nanotubes: applied for determination of holmium content in biological and environmental samples. Mater. Sci. Eng. C 30, 555–560 (2010)
Ganjali, M.R., Aghabalazadeh, S., Rezapour, M., Hosseini, M., Norouzi, P.: La3+ Carbon nanotube (CNT) based electrode using a new (1-[({2-[2-(2-hydroxy-1-naphtyl)-3-(2-{[(E)-1-(2-hydroxy-1-naphtyl)methylidene]amino}ethyl)-1-imidazolidinyl]ethyl}imino)methyl]-2-naphthol). Int. J. Electrochem. Sci. 5, 1743–1753 (2010)
Zamani, H.A., Rajabzadeh, G., Ganjali, M.R., Norouzi, P.: Determination of gadolinium(III) ions in soil and sediment samples by a novel gadolinium membrane sensor based on 6-methyl-4-{[1-(2-thienyl)methylidene]amino}3-thioxo-3,4-dihydro-1,2,4-triazin-5-(2H)-one. Anal. Chim. Acta 598, 51–57 (2007)
Ganjali, M.R., Norouzi, P., Faridbod, F., Hajiabdollah, N., Dinarvand, R., Meghdadi, S.: Lutetium(III) ions determination in biological and environmental samples by a lutetium(III) sensor based on N, N0-bis (2-Pyridinecarboxamide)-1,3-benzene as a sensing material. Anal. Lett. 41, 3–23 (2008)
Ganjali, M.R., Norouzi, P., Farrokhi, R., Faridbod, F., Larijani, B., Meghdadi, S.: Lanthanide recognition: determination of thulium(III) ions in presence of other rare earth elements by a thulium(III) sensor based on 4-methyl-1,2-bis (2-pyridinecarboxamido) benzene as a sensing material. Anal. Lett. 41, 2322–2343 (2008)
Zamani, H.A., Ganjali, M.R., Seifi, N.: Dysprosium(III) ion-selective electrochemical sensor based on 6-Hydrazino-1,5-diphenyl-6,7-dihydropyrazolo[3,4-d]pyrimidine-4(5H)-imine. Collect. Czech. Chem. Commun. 72, 1189–1206 (2007)
Ganjali, M.R., Norouzi, P., Ghorbani, H., Larijani, B., Tadjarodi, A., Hanifehpour, Y.: Lanthanide recognition: fabrication of an ytterbium PVC membrane sensor based on N-(6-picolyl)-N′-(4-metoxyphenyl) thiourea(PCMPT). Can. J. Anal. Sci. Spectros. 51, 279 (2006)
Behmadi, H., Zamani, H.A., Ganjali, M.R., Norouzi, P.: Determination of neodymium(III) ions in soil and sediment samples by a novel neodymium(III) sensor based on benzyl bisthiosemicarbazone. Electrochim. Acta 53, 1870–1876 (2007)
Ganjali, M.R., Ahmadalinezhad, A., Norouzi, P., Adib, M.: Nd(III)-PVC membrane sensor based on 2-{[(6-aminopyridin-2-yl)imino]methyl}-phenol. J. Appl. Electrochem. 36, 931–936 (2006)
Ganjali, M.R., Akbar, V., Ghorbani, M., Norouzi, P., Ahmadi, A.: Fluoride determination in some mouth wash preparations by a novel La(III) graphite coated membrane sensor based on amitraz. Anal. Chim. Acta 531, 185–191 (2005)
Ganjali, M.R., Gholivand, M.B., Rahimi-Nasrabadi, M., Maddah, B., Salavati-Niasari, M., Ahmadi, F.: Synthesis of a new octadentates schiff’s base and its application in construction of a highly selective and sensitive lanthanum (III) membrane sensor. Sens. Lett. 4, 1–8 (2006)
Ganjali, M.R., Norouzi, P., Alizadeh, T., Tajarodi, A., Hanifehpour, Y.: Fabrication of a highly selective and sensitive Gd(III)-PVC membrane sensor based on N-(2-pyridyl)-N′-(4-nitrophenyl)thiourea. Sens. Actuators B 120, 487–493 (2007)
Ganjali, M.R., Norouzi, P., Daftari, A., Faridbod, F., Salavati-Niasari, M.: Fabrication of a highly selective Eu(III) membrane sensor based on a new S-N hexadentates Schiff’s base. Sens. Actuators B 120, 673–678 (2007)
Ganjali, M.R., Faridbod, F., Norouzi, P., Adib, M.: A novel Er(III) sensor based on a new hydrazone for the monitoring of Er(III) ions. Sens. Actuators B 120, 119–124 (2006)
Faridbod, F., Ganjali, M.R., Larijani, B., Norouzi, P.: Multi-walled carbon nanotubes (MWCNTs) and room temperature ionic liquids (RTILs) carbon paste Er(III) sensor based on a new derivative of dansyl chloride. Electrochim. Acta 55, 234–239 (2009)
Ganjali, M.R., Davarkhah, N., Ganjali, H., Larijani, B., Norouzi, P., Hossieni, M.: A novel europium(III) sensor based on 4E-4-(2-phenylviazenyl)-2-((E)-(2-aminoethylimino)methyl)phenol. Int. J. Electrochem. Sci. 4, 762–771 (2009)
Shamsipur, M., Hosseini, M., Alizadeh, K., Talebpour, Z., Mousavi, M.F., Ganjali, M.R., Arca, M., Lippolis, V.: PVC membrane and coated graphite potentiometric sensors based on Et4todit for selective determination of samarium(III). Anal. Chem. 75, 5680–5686 (2003)
Zamani, H.A., Arvinfar, A., Rahimi, F., Imani, A., Ganjali, M.R., Meghdadi, S.: Praseodymium analysis in aqueous solution by Pr3+–PVC membrane sensor based on N,N′-bis(4-hydroxysalicylidene)-1-3-phenylenediamine. Mater. Sci. Eng. C 31, 307–312 (2011)
Ganjali, M.R., Memari, Z., Faridbod, F., Norouzi, R., Dinarvand, P.: Sm3+ potentiometric membrane sensor as a probe for determination of some pharmaceutics. Electroanalysis 20, 2663–2670 (2008)
Ganjali, M.R., Tavakoli, M., Faridbod, F., Riahi, S., Norouzi, P., Salavati-Niassari, M.: Interaction study of a new bis-bidentate schiff’s base with some metal ions and its application in fabrication of Sm(III) potentiometric membrane sensor. Int. J. Electrochem. Sci. 3, 1559–1573 (2008)
Ganjali, M.R., Norouzi, P., Atrian, A., Faridbod, F., Meghdadi, S., Giahi, M.: Neutral N, N′-bis(2-pyridinecarboxamide)-1,2-ethane as sensing material for determination of lutetium(III) ions in biological and environmental samples. Mater. Sci. Eng. C 29, 205–210 (2009)
Ganjali, M.R., Norouzi, P., Shamsolahrari, L., Ahmadi, A.: PPb level monitoring of lanthanium by a novel PCV-membrane sensor based on 4-methyl-2-hydrazinobenzothiazole. Sens. Actuators B 114, 713–719 (2006)
Zamani, H.A., Ganjali, M.R., Faridbod, F.: A lutetium PVC membrane sensor based on (2-oxo-1,2–diphenylethylidene)-N-phenylhydrazinecarbothioamide. J. Serb. Chem. Soc. 76, 1295–1305 (2011)
Hypercube Inc.: Hyperchem, v.7.5. Hypercube Inc. (2003) http://www.hyper.com
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F., Stewart, J.J.P.: AM1: a new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 107, 3902–3909 (1985)
Todeschini, R., Consonni, V., Mauri, A., Pavan, M.: DRAGON-Software for the calculation of molecular descriptors. Version 4.0 for Windows. Talete SRL, Milan (2004)
Todeschini, R., Consonni, V., Mauri, A., Pavan, M.: Detecting “bad” regression models: multicriteria fitness functions in regression analysis. Anal. Chim. Acta 515, 199–208 (2004)
Hammer, M.C., Steinhauer, V., Gasteiger, J.: Deriving the 3D structure of organic molecules from their infrared spectra. Vib. Spectrosc. 19, 151–164 (1999)
Consonni, V., Todeschini, R., Pavan, M.: Structure/response correlations and similarity/diversity analysis by GETAWAY descriptors. 1. Theory of the novel 3D molecular descriptors. J. Chem. Inf. Comput. Sci. 42, 682–692 (2002)
Consonni, V., Todeschini, R., Pavan, M., Gramatica, P.: Structure/response correlations and similarity/diversity analysis by GETAWAY descriptors. 2. Application of the novel 3D molecular descriptors to QSAR/QSPR studies. J. Chem. Inf. Comput. Sci. 42, 693–705 (2002)
Tropsha, A., Gramatica, P., Gombar, V.K.: The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb. Sci. 22, 69–77 (2003)
Golbraikh, A., Tropsha, A.: Beware of q2! J. Mol. Graph. Model 20, 269–276 (2002)
Netzeva, T.I., Worth, A.P., Aldenberg, T., Benigni, R., Cronin, M.T.D., Gramatica, P., Jaworska, J.S., Kahn, S., Klopman, G., Marchant, C.A., Myatt, G., Nikolova-Jeliazkova, N., Patlewicz, G.Y., Perkins, R., Roberts, D.W., Schultz, T.W., Stanton, D.T., van de Sandt, J.J.M., Tong, W., Veith, G., Yang, C.: Current status of methods for defining the applicability domain of (quantitative) structure-activity relationships: the report and recommendations of ECVAM workshop 52. Altern. Lab. Anim. 33, 155–173 (2005)
Eriksson, L., Jaworska, J., Worth, A.P., Cronin, M.T.D., McDowell, R.M., Gramatica, P.: Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health Perspect. 111, 1361–1375 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiani-Anbouhi, R., Ganjali, M.R. & Norouzi, P. Application of QSPR for prediction of the complexation stabilities of Sm(III) with ionophores applied in lanthanoid sensors. J Incl Phenom Macrocycl Chem 81, 441–450 (2015). https://doi.org/10.1007/s10847-014-0472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0472-9