Abstract
Appropriate sampling methods are essential for monitoring and managing all wildlife. There is limited information on how to monitor ground-dwelling invertebrates in the alpine zone compared to more commonly studied lower-elevation habitats. We aim to understand the strengths, weaknesses, and cost-effectiveness of invertebrate survey methods when deployed in the alpine zone. We tested four methods on the South Island of New Zealand (Aotearoa): pitfall traps, tracking tunnels, visual surveys, and trail cameras. We focused on methods for ground-dwelling large-bodied invertebrates, a group most likely to be impacted by introduced mammalian predators. Pitfall traps and trail cameras were practical methods for surveying alpine invertebrates. Tracking tunnels only reliably detected large wētā (Orthoptera: Anostostomatidae), as other invertebrates were too light to leave distinguishable tracks. Visual surveys did not generate enough observations to be considered a successful method. We found no difference in the total abundance or easily recognised taxonomic groups detected by pitfall traps and trail cameras. Trail cameras detected fewer wētā compared to tracking tunnels and pitfall traps. Of the successful methods, pitfall traps were the most cost-effective, followed by tracking tunnels and trail cameras. Tracking tunnels were the fastest to install and required the least processing time, followed by pitfall traps and trail cameras. We recommend using pitfall traps to survey large-bodied alpine ground-dwelling invertebrates to better understand population trends or community composition. This method was consistent across all alpine habitat types and has the potential to be an effective method to inform conservation monitoring and management programmes.
Implications for insect conservation
Monitoring is vital to understand how invertebrates respond to change and evaluate conservation management program outcomes. This study provides practical information on potential monitoring tools for invertebrates in the alpine zone and recommends pitfall traps to survey large-bodied alpine invertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robust, standardised, and long-term data inform conservation practitioners on which species need management and which management actions generate the desired outcomes (Lindenmayer et al. 2012). A major issue with how we collect data on invertebrates is the sheer number and design of available surveying methods. Depending on the environment and target group or species, researchers may use malaise traps (Schmidt et al. 2019), pan traps (Popic et al. 2013), pitfall traps (Perner and Schueler 2004), vacuum sampling (Doxon et al. 2011), soil sampling (Schneider et al. 2019), sweep netting (Doxon et al. 2011) or artificial refuges (Bowie et al. 2014), among others. Once a method is selected, sampling design can also vary widely. Pitfall traps, for example, can be made of metal, glass, or plastics of various colours. These differences can introduce biases towards the trap’s effectiveness for surveying particular species or groups (Lang 2000; Buchholz and Möller 2018). The number of traps and their layout can also influence how many invertebrates are caught and what components of the invertebrate community are sampled (Engel et al. 2017). All these differences in methodology, trap design, and layout make comparing studies challenging. Furthermore, the large number of available methods make selecting the right one difficult, especially when there may be limited information on how they work in your target habitat.
Like all research fields, certain species or environments in the terrestrial invertebrate literature are more represented than others. Flying pollinators, like butterflies (van Swaay et al. 2008; Wepprich et al. 2019; Lee et al. 2020) or bees (Meiners et al. 2019; Portman et al. 2020; Cane 2021), are frequently examined groups, and there are multiple examples of well developed, long term monitoring protocols for these invertebrates (Kammerer et al. 2020; Cane 2021; Culbertson et al. 2022). For ground-dwelling invertebrates, there are good examples of how to monitor arthropod communities in grassland habitats (Standen 2000; Zaller et al. 2015; Buchholz and Möller 2018). Invertebrate monitoring strategies in forest communities, especially tropical forests, are well documented, where methods like visual surveys and pitfall traps are effective (Cardoso et al. 2008; Campanaro and Bardiani 2012; Riley et al. 2016). Studies on monitoring strategies for terrestrial arthropods in the alpine zone are less common (e.g. Schneider et al. 2019; Paler et al. 2021). This is concerning because as our climate warms, alpine species face a growing number of threats, including habitat loss and fragmentation (Jackson et al. 2015) and changes in the seasonality of resources which may disrupt the life cycle of alpine flora and fauna (Ernakovich et al. 2014). As a result, a dedicated effort is required to develop effective methods in alpine areas to better monitor and conserve alpine invertebrates.
In New Zealand, the alpine zone is expansive, covering around 11% of the total land area (~ 29,482 km²) and hosts an extensive suite of endemic invertebrates, including, but not limited to, spiders, beetles, flies, wasps, moths, wētā, grasshoppers, and giant carnivorous snails (O’Donnell et al. 2017). Many of these species are alpine specialists, while others have likely been lost from lower elevations due to extreme predation pressures. There are many potential threats to invertebrates in the New Zealand alpine zone, including invasive plants (Litt et al. 2014) and climate change (Chinn and Chinn 2020), but we focus on surveying methods that could help identify the impacts of invasive mammalian predators. Invasive predators are a known threat to native fauna in New Zealand and have devastated native populations at lower elevations (Angel et al. 2009; Ruscoe et al. 2013; Goldson et al. 2015; O’Donnell et al. 2017). In alpine regions, the most notable invasive predators of invertebrates are stoats (Mustela erminea) and mice (Mus musculus) (Smith et al. 2005; Wilson and Lee 2010). We focus on testing potential monitoring methods for large-bodied ground-dwelling invertebrates (which we define as greater than or equal to 1 cm in body length) because the combination of their relatively large size and slow locomotion makes large-bodied invertebrates particularly sensitive to predation by introduced predators.
We compared four potential monitoring methods that are particularly suited to surveying large-bodied terrestrial invertebrates: pitfall traps, visual surveys, tracking tunnels, and trail cameras. Pitfall traps have been used to monitor numerous arthropod groups across a wide array of habitats, and this method is regarded as the most efficient method for time and effort to sample ground-dwelling taxa in lowland temperate habitats (Skvarla et al. 2014; Gobbi et al. 2018). The biases associated with pitfall traps are well documented. Pitfalls often over-represent large mobile invertebrates, and trap material, preservative type, shape, and placement, all influence the number and type of species collected (Luff 1975; Uetz and Unzicker 1975; Digweed 1995; Skvarla et al. 2014; Buchholz and Möller 2018; Hohbein and Conway 2018).
Visual surveys generate counts of invertebrates as researchers search for invertebrates along a pre-determined transect. This method can sample flying, arboreal and ground-dwelling invertebrates and can be biased towards large invertebrates that are easy to see and identify (Zaller et al. 2015). Results are limited to species that are active when the search is conducted and are partly dependent on the individual skill and experience of the collectors (Bouget et al. 2008; Riley et al. 2016). Visual surveys are effective in warm tropical environments, like rainforests, that promote high activity levels for invertebrates (Gobbi et al. 2018). However, visual survey detections are more variable in cooler environments, like New Zealand’s low-elevation forests. For example, Watts et al. (2011) found surveys to be less successful than passive surveying methods, like tracking tunnels, while Carpenter et al. (2016) found visual surveys to sample equally as well as pitfall traps.
Researchers use tracking tunnels to generate presence-absence data by luring invertebrates across an inked card with a centrally placed bait causing them to leave tracks as they exit the device. In New Zealand, tracking tunnels are a common method used to survey introduced predators like rodents and stoats (Brown et al. 1996; Blackwell et al. 2002; Kemp et al. 2022) and have also been shown to successfully survey large wētā (Anostostomatidae, a group of native Orthoptera common in New Zealand) that leave easily identifiable tracks (Watts et al. 2008, 2011; Carpenter et al. 2016). To our knowledge, tracking tunnels have not been tested on invertebrates in the alpine zone, but they have potential as a survey method in this environment.
Time-lapse trail cameras generate count data of invertebrates by taking a picture of the ground at a pre-determined time interval. Trail cameras have predominantly been used to survey large mammals and birds (O’Brien and Kinnaird 2008; Burton et al. 2015) but have also been used to survey smaller organisms like lizards (Gibson et al. 2015; Bertoia et al. 2021) and invertebrates (Potter et al. 2021). Trail cameras can sample any invertebrate that comes into the frame but are biased towards larger, slower invertebrates that are easier to see and remain in the frame long enough to be photographed (Collett and Fisher 2017). More complex habitats may limit what can be observed on trail camera photographs as vegetation, rocks or subsurface retreats may hide invertebrates from the camera’s field of view (Collett and Fisher 2017).
Previous studies comparing invertebrate surveying methods have shown trail cameras to be effective in low-elevation forest and grassland habitats. Cameras outperformed pitfall traps and captured images and video of invertebrates escaping or avoiding traps (Zaller et al. 2015; Collett and Fisher 2017). Research comparing pitfall trapping and hand searching is often site or species-specific. For example, hand searches, where researchers spent 15–20 min looking under stones and hiding places for carabid beetles, slightly outperformed pitfall traps in the high Andes mountains in Chile (Gobbi et al. 2018). However, pitfall traps were more successful than quadrat sampling for sampling the ground-dwelling invertebrate community in dry riverbeds in New Zealand (Corti et al. 2013). The effectiveness of invertebrate surveying methods depends on the environment and community being surveyed. As a result, we tested potential monitoring methods for ground-dwelling large-bodied alpine invertebrates to assess how multiple methods work in the alpine zone.
We aimed to understand the strengths, weaknesses, and cost effectiveness of the four survey methods for ground-dwelling large-bodied invertebrates in the alpine zone. We also wanted to know if these four methods are equally effective at detecting invertebrates across different habitat types common in alpine areas (e.g., tussock fields, rocky fields, and low-lying shrubs). Based on the previous research described above, we predicted that pitfall traps would successfully sample the large-bodied invertebrate community in tussock and shrub habitats but may be less effective in rocky terrain where it may be hard to install pitfalls flush with the ground (Wynne et al. 2019). We expect trail cameras and visual surveys to be less effective in more complex habitats, like shrubby sites where vegetation may block the camera’s field of view or allow invertebrates to hide from observers (Collett and Fisher 2017). Lastly, we expect tracking tunnels to be equally effective in all habitat types, but observations will be limited to wētā tracks as this method has mostly been explored for use on large wētā (Watts et al. 2008, 2011; Carpenter et al. 2016). We expect visual surveys will be the most cost-effective method, followed by pitfall traps, tracking tunnels, and trail cameras.
Methods
Study area
This study occurred in Fiordland National Park in the southwest of the South Island of Aotearoa, New Zealand (hereafter, ‘Fiordland’). Our field sites encompass the Homer and Gertrude Valleys, ranging from 900 to 1,100 m above sea level (a.s.l). Fiordland experiences intermittent snowfall from May-October, with the occasional snowfall during the austral summer.
Typical alpine habitat in New Zealand consists of three major types: tussock grasslands, shrub fields, and rock fields. Rock fields were generally 60–80% rock covered, ranging from small pebbles to scree fields and large boulders. The rocks were interspersed with sparse vegetation consisting of mosses, small ferns, and mountain buttercup (Ranunculus lyallii). Tussock grasslands were comprised of 50–60% red tussock (Chionochloa rubra), narrow-leafed snow tussock (C. rigida), and fescue tussock (Festuca novae-zelandiae), with the remaining vegetation consisting of mosses, mountain buttercup, dracophyllum (Dracophyllum rosmarinifolium) and common speargrass (Aciphylla squarrosa). Shrub fields were dominated by woody shrubs around 1 m tall, including hebes (e.g., Veronica hectorii) and dracophyllum. The shrub habitat contained a variety of other native plants like mountain daisies (Celmisia spp.), mountain buttercup, and common speargrass, with various ferns and mosses.
Study design
We tested potential monitoring methods in all three habitat types (tussock fields, areas of low-lying shrubs, and rocky fields) in Fiordland with four replicates per habitat type giving 12 sites in total. We established three 90 m long parallel transects at each site spaced 10 m apart based on the minimum width and length of natural habitat patches. This enabled each of the methods requiring device spacing (pitfall traps and tracking tunnels) and the visual survey transect to be on independent transects. A single camera (point sample) was allocated to one of these transects (see below). All transects had a random starting point and bearing within the limitations imposed by the size of the habitat type and surrounding terrain. We placed our field sites at least 30 m apart to limit the chances of catching the same invertebrate in multiple lines, this distance is further than radio-tracked Deinacrida heteracantha, a species of wētā larger in size than the large-bodied invertebrates that occupy this habitat, are known to travel in one night (Watts and Thornburrow 2011; Fig. 1). At each site, pitfall traps, tracking tunnels, and visual surveys were randomly assigned to one transect. Furthermore, we used nine trail cameras and placed a single camera at the 45 m mark of three out of the four pitfall trap transects per habitat (three cameras surveyed each habitat type). We baited pitfall traps, tracking tunnels, and trail cameras with Pams® Smooth Peanut Butter (Pams Products Ltd, New Zealand), a bait commonly used to attract mice and invertebrates (Watts et al. 2008, 2011; Nathan et al. 2013; Carpenter et al. 2016). We surveyed field sites once in January, February, March/early April, and April/early May 2021. During each sampling trip, all 12 sites were visited once. We baited each device in the morning or early afternoon before running the visual search transects. The following day, we identified the catch from the pitfall traps, collected the tracking tunnel cards and shut down the site (all bait removed, and pitfall traps inverted and covered to prevent insects or lizards from being caught between sampling sessions and to avoid attracting predators). We checked the camera batteries each time we closed the site and collected the memory cards at the end of the season.
Map of our field site showing the location of all 12 transects. Diamonds represent sites where all four methods were tested while circles represent sites where cameras were absent. The inset represents an example site layout of the nine sites where all four methods were present. These nine sites contained a transect for tracking tunnels (blue line), pitfall traps (red line) and visual searching (black line), and a trail camera (white dot). Sites were at least 30 m apart
Pitfall traps
Pitfall trap transects consisted of 10 pitfall traps that we placed at 10 m intervals. We made pitfall traps from 1 L transparent plastic bottles with a 90 mm diameter opening. The top quarter of each bottle was removed, inverted, and placed back onto the bottle to create a transparent funnel with the narrow end of the funnel measuring 2.5 cm in diameter. We then covered the pitfall traps with 18 × 18 cm white plastic covers (3 mm polypropylene sheet) to shield the trap from rain and falling debris. We based the pitfall trap design on Hohbein and Conway (2018), who proposed a standardised design after reviewing 257 pitfall studies (Fig. 2). Transparent traps limit the effect of trap colour on capture rates, while funnels decrease the number of invertebrate escapes and reduce vertebrate bycatch (Hohbein and Conway 2018). Because pitfall traps had the potential to catch threatened native lizards, we filled the pitfalls with ~ 2 cm of water rather than a preserving liquid. We did not catch any lizards in our pitfall traps, but if we had, we would have been able to release them with minimal harm. To set the traps, we spread ~ 5 g of peanut butter bait on the underside of the cover over the trap opening and fashioned a wire stand to hold the cover approximately 2 cm above the ground. We were not using a preserving liquid, so we checked the pitfall traps after 24 h. We used such a short trapping duration to make pitfall traps comparable with the tracking tunnels, which are often replaced daily when surveying rodents and invertebrates (Blackwell et al. 2002; Watts et al. 2011). We also wanted to limit any effect that stale bait may have on capture rates and reduce the possible effects that early captures may have on later captures.
During the first survey, we kept all the large-bodied invertebrates for identification. We counted the number of individuals caught, measured their body length (front of the head to the tip of the abdomen) to the nearest millimetre using a 15 cm ruler, and identified each to the family level except for centipedes, which we identified to the order level. On subsequent trips, we only kept individuals that were not observed in the first monitoring session. This limited the number of invertebrates killed during the study.
Camera trapping
Cameras (Reconyx HC 500 Hyperfire, REconyx Inc., Holmen) were programmed to take a photograph every 15 min, an interval shown to adequately survey invertebrates without taking excessive images (Collett and Fisher 2017). We attached the cameras 60 cm off the ground to a 1 m black steel pole with the camera lens facing the ground. We then placed a 30 cm ruler in the field of view as a scale to measure the length of invertebrates in the pictures. Depending on the habitat, we secured the rulers with an all-surface adhesive (THE ONE®, Selleys, New Zealand) or wire. We placed ~ 5 g of peanut butter bait in the camera’s view above the ruler so that invertebrates attracted to the bait would be in the picture. At the end of this 24-hour period, we removed peanut butter bait from the cameras.
We used Timelapse2 (Greenberg and Godin 2015), an image-analysis programme that simplifies the data collection from trail camera images, to generate data from the images. Timelapse2 extracts metadata from the images (time and camera ID) and allows the user to create a custom interface for entering data by typing, selecting from a custom list, or clicking on objects in the image to count them (Greenberg and Godin 2015). We counted all individual invertebrates larger than 1 cm, identified each to family level where possible, and summed observations after 24 h of operations. To limit recounting invertebrates, individuals in the same or very similar position (e.g., in the same position eating peanut butter) on consecutive pictures were not recounted. As a result, there is some risk of double-counting invertebrates, but we did our best to minimise it given the quality of the images we collected.
Visual surveys
We conducted visual surveys of the ground 50 cm on either side (i.e., a one-metre strip) along the length of the 90 m transect and aimed to spend no more than 15 min on each search. Searches were limited to 15 min to make them a realistic tool for surveying remote alpine areas where limited site access and adverse weather conditions can make time a limiting factor. Other surveys of large-bodied invertebrates in New Zealand are similarly short (e.g., Schori et al. 2020), so we felt that 15 min was sufficient. Surveys were only completed during the day, as running night searches and the other survey methods was logistically impossible. The observer would move slowly along the transect, looking at the ground underneath surface vegetation, but we did turn any rocks or other surface retreats. When we observed an invertebrate, we identified it to the family level (if possible) and estimated body length by eye (if the invertebrate remained in the open long enough to do so). The same single observer conducted all our visual surveys for this study.
We completed a small test to see whether we should conduct night visual surveys. We ran three surveys from 12:00–1:30 am in early December, on which we observed a total of three carabid beetles. The relatively low level of insect activity combined with the logistic difficulty of a small team running surveys by night while maintaining the other methods in the experiment by day caused us to forgo the night surveys.
Tracking tunnels
We used standard Black Trakka™ tracking tunnels (500 × 100 × 100 mm; Gotcha Traps, Warkworth, New Zealand), a commonly used method to survey introduced pests and wētā at lower elevations in New Zealand (King and Scurr 2013; Stringer et al. 2014). Tracking tunnel transects consisted of 10 tunnels placed at 10-metre intervals. During surveys, we placed ~ 5 g of peanut butter directly onto the ink in the centre of the tracking card.
Temperature and weather data
The Milford Road Alliance provided hourly temperature and precipitation data for our field sites which were collected at the East Homer Road weather station. This station is in the Upper Hollyford Valley at the base of Mt Talbot 500 m southeast of the eastern Homer Tunnel entrance in Fiordland National Park on the South Island of New Zealand (-44.764011, 167.995403, 870 m a.s.l). This weather station is 250 m from the closest survey site, 1.9 km from the furthest and within 30 m of elevation.
Statistical analysis
We first compared all survey methods by summarising the total invertebrate abundance and diversity of recognisable taxonomic units by field site and habitat type. We also calculated the cost of equipment, time to set up, and time required to process the data generated by each method. We undertook statistical analysis to compare pitfall traps, trail cameras, and tracking tunnels, but more data needed to be obtained from visual surveys to include them in the analysis. We combined observations from the ten pitfall traps and ten tracking tunnels, respectively, to the site level per trip. We pooled the data in this way as individual traps and tunnels along the same transect were not spatially independent. As we only had one camera per site, these data were already at the site level. We completed all analyses in RStudio (RStudio Team 2020).
We used a generalised additive mixed model (GAMM) with a negative binomial distribution using the package mgcv (Wood 2011) to see whether similar numbers of large-bodied invertebrates were observed via pitfall trapping and trail cameras across the three habitat types. We only had access to nine trail cameras, so our design was slightly unbalanced (models contained 12 sites with pitfall traps and 9 sites with cameras). The GAMM contained counts of invertebrates as the response variable, with survey method (pitfall traps or trail cameras), habitat type (shrub, tussock, or rock) as categorical variables, and collection date (recorded as the number of days after January 1st, 2021) as a smoothed predictor variable. We also included an interaction effect between survey method and habitat type to test for differences in the number of large-bodied invertebrates observed by each method in each habitat type. As we revisited the same sites across the summer season, site number was included as a random effect to allow for observations of invertebrates to vary across site. We also tested model variants including minimal and/or maximal temperatures but these variables were eventually omitted as they were too highly correlated with collection date. Invertebrate counts were generated from 84 replicates (12 lines of pitfall traps and nine trail cameras visited four times across the summer season).
We used data for each taxonomic group generated by the trail cameras and pitfall traps to analyse differences in community composition at the family level. We used a permutational multivariate analysis of variance (PERMANOVA, 999 permutations; Anderson 2001) based on the Bray-Curtis Dissimilarity index to examine differences in the invertebrate community as detected by method and at each habitat type. Unbalanced designs can make a PERMANOVA sensitive to differences in dispersions among groups (Anderson 2014), so we only included the nine sites where all four methods were present. Furthermore we tested for homogeneity of multivariate dispersion using a permutational analysis of multivariate dispersion (PERMDISP, Anderson 2001). We completed a PERMDISP in conjunction with a PERMANOVA to ensure that observed significant differences result from differences between mean values of group centroids rather than within-group variability from the centroids (Anderson 2006). To visualise differences in community composition between survey methods and habitat types, we used non-metric multidimensional scaling (NMDS) with a Bray-Curtis Dissimilarity index to generate minimum convex hulls. We conducted this analysis using the vegan package (Oksanen et al. 2020).
Because tracking tunnels only recorded wētā activity, we ran a separate GAMM with a binomial distribution for wētā data only. We converted wētā abundance data, generated by the pitfall trap lines and trail cameras, to the presence of wētā per transect, so that they could be compared with presence-absence data generated by tracking tunnels. The GAMM had 132 replicates from the 12 lines of pitfall traps and tracking tunnels with the nine trail cameras each visited four times during the season. The binomial GAMM had the same model makeup used in the abundance data analysis (see above) but without the interaction effect.
Results
Overview
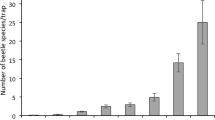
We collected 787 observations of invertebrates from the three methods that generate abundance data. This resulted in 213 observations from nine trail cameras, 551 observations from twelve transects of pitfall traps, 23 observations from the twelve transects of visual surveys, and 52% of tracking all devices tracked wētā. We detected eight families from seven orders (See supplementary materials for abundances for each order; Fig. 3), with Carabidae, Lycosidae and Anostostomatidae being the most common families. We detected the highest abundance of invertebrates in all three habitat types with pitfall traps, followed by cameras and visual surveys. We also detected the greatest number of recognised taxonomic groups in all three habitat types using pitfall traps, followed by cameras, and then visual surveys (Fig. 3). During the sampling period, temperatures in Fiordland were quite similar for January, February, and March, with February having the highest mean (18.83 °C ± 0.27) but only by ~ 2 °C. May was cooler than the other three months, having the lowest average, absolute minimum, and absolute maximum temperatures by upwards of 5 °C.
Total abundance of each invertebrate family detected by nine trail cameras, 120 pitfall traps and 12 transects of visual surveys run during the 2020-21 austral summer season in Fiordland, New Zealand. (A) represents detections in shrubby habitat (n = 354), (B) represents rocky habitat (n = 231), and (C) represents tussock habitat (n = 202)
Invertebrate abundance
Due to the limited number of observations from visual surveys, we removed them from the analysis. This resulted in 551 invertebrates sampled by pitfall traps and 213 from trail cameras to be used in the analysis (764 large-bodied invertebrates in total). Over the summer season, we collected a total of 2,997 images with our trail cameras. We omitted 784, leaving 2,213. Of the 784 images we removed, 508 were removed due to a technical issue that caused the photo quality to be too poor; 144 images were omitted because the pictures were obscured by rain on the lens or vegetation that had grown into the camera’s field of view.
We detected no difference in invertebrate abundance as recorded by pitfall traps and trail cameras (χ2(1) = 0.65, p = 0.42). We also found no significant difference in the number of invertebrates observed across the three habitat types (χ2(2) = 3.78, p = 0.15). Lastly, there were no significant differences in the interaction between method and habitat type (χ2(2) = 1.64, p = 0.44. For the full model output, see supplementary materials S1.
Invertebrate community composition
There was no difference in dispersion between methods or habitat types (PERMDISP Table 1). This suggests that the results from the PERMANOVA represent differences in community composition and not within-group variability. We found no significant difference in community composition as detected by pitfall traps compared with trail cameras (R2 = 0.03; Table 1; Fig. 4). We detected a significant difference in the invertebrate communities among habitat types, although the amount of variation explained was relatively small (R2 = 0.09; Table 1;Fig. 5). Furthermore, a post hoc comparison with Holm’s correction showed no significant difference in community composition between any habitat types, suggesting that the differences in community composition between habitat types are small (Table 1).
NMDS ordination of large-bodied terrestrial invertebrate groups as measured by trail cameras and pitfall traps in Fiordland, New Zealand during the austral summer of 2020-21 (k = 2). Red polygons represent invertebrate groups detected by pitfall traps and blue polygons represent groups detected by trail cameras. All polygons are convex hulls. There was no significant difference in the invertebrate community as detected by pitfall traps or trail cameras
NMDS ordination of large-bodied terrestrial invertebrate groups as measured by trail cameras and pitfall traps across habitat types in Fiordland, New Zealand during the austral summer of 2020-21 (k = 2). The grey polygon represents groups detected in rocky habitats, the green polygon represents shrubby habitats, and the yellow polygon represents tussock habitats. All polygons are convex hulls. There was a significant difference in the invertebrate community inhabiting shrub and tussock habitats (p = 0.006)
Wētā detection comparison
The detection rate of wētā was 63% for tracking tunnels, 65% for pitfall traps and 39% for cameras. No wētā were recorded on visual surveys (Fig. 2). We found a slight significant difference in wētā detection rates cameras across the three methods (χ2(2) 6.15, p = 0.05; Fig. 6). There was also a significant difference in detection rates between habitat types (χ2(2) = 13.30, p = 0.001; Fig. 6). Pairwise Tukey post hoc tests showed trail cameras detected fewer wētā than pitfall traps (t(118) = -2.32, p = 0.05). There was, however, no difference in wētā detections between cameras and tracking tunnels (t(118) = -2.11, p = 0.09) nor tracking tunnels and pitfall traps (t(118) = -0.26, p = 0.96). Additionally, wētā detections were significantly higher in shrubby habitats compared to rocky terrain(t(118) = -3.53, p = 0.002) and tussock fields (t(118) = 2.97, p = 0.01). There was no difference in wētā detections between rocky and tussock habitats (t(118) = -0.73, p = 0.75). For the full model output, see supplementary materials S1.
Proportions of lines with at least one wētā detection by pitfall traps, tracking tunnels and trail cameras in Fiordland. Proportions are calculated from 120 tracking tunnels and 120 pitfall traps and nine cameras that were sampled across the 2020-21 austral summer season. Trail cameras took a photograph every 15 min resulting in 2,213 images
Costs and processing time
Visual surveys were the cheapest method, followed by pitfall traps, tracking tunnels and trail cameras (Table 3). Visual surveys were also the fastest method to install, followed by tracking tunnels and trail cameras, with pitfall traps having the longest installation time. Lastly, visual surveys took the least amount of time to process, followed by tracking tunnels, pitfall traps and trail cameras (Table 2).
Discussion
Overall, pitfall traps, and trail cameras sampled large-bodied ground-dwelling invertebrates that are commonly found in the New Zealand alpine zone and have the potential to be used as monitoring methods. Tracking tunnels only reliably sampled wētā and visual surveys did not generate enough data to be considered a viable surveying method. Pitfall traps and trail cameras performed consistently across the three habitat types assessed. However, cameras were considerably more expensive than all other methods assessed and took more time to generate data from the images. Tracking tunnels detected a similar amount of wētā activity as pitfall traps, and slightly more than trail cameras, but did not provide data on the abundance or diversity of the large-bodied invertebrate community. This suggests that tracking tunnels may be more useful as a specialist method to survey wētā, or similarly large Orthopterans, than one used to survey the large-bodied invertebrate community. Tracking tunnels were more expensive than pitfall traps but slightly faster to set up and process. When comparing wētā activity across habitat types, trail cameras detected fewer wētā compared to tracking tunnels and pitfall traps. Generally, wētā were more active in shrubby areas than tussock or rocky terrain.
Successful potential monitoring methods
We successfully surveyed the large-bodied alpine invertebrate community using pitfall traps and trail cameras, but pitfall traps have a few advantages that make them more effective. Though the difference was not significant, pitfall traps sampled a more comprehensive community than trail cameras, capturing species like amphipods and centipedes that were less common at our field sites. Pitfall traps also sampled more wētā. These slight differences may be due to sampling effort as we deployed more pitfall traps than trail cameras. Alternatively, these invertebrates could be more challenging to detect using cameras as they may spend more time under surface cover outside the camera’s field of view. Overall, pitfall traps and trail cameras sampled a similar large-bodied invertebrate community across all habitat types suggesting that either method could be a potential monitoring method.
To synthesise our findings, we have compiled a list of each method’s strengths, weaknesses, and limitations, with references (Table 3). As well as allowing us to sample the large-bodied alpine invertebrate community, pitfall trapping has the additional advantage of allowing for specimen collection. We know very little about large-bodied alpine invertebrates, and specimens can be identified to the species level, used in genetic analysis (Gurdebeke and Maelfait 2002), or be shared with other researchers aiding in the amount of knowledge generated by a single survey. The use of pitfall traps also avoids issues that trail cameras suffer, like technical difficulties, the inability to identify some individuals, and the potential to double-count invertebrates. We lost data to technical difficulties with our trail cameras during our February field trip, while the pitfall traps functioned properly for the entire field season. These added benefits and their overall effectiveness in all alpine habitats give pitfall traps the edge over trail cameras for surveying large-bodied invertebrates in Fiordland.
Cost-effective potential monitoring methods
Pitfall traps were also the most cost-effective method compared to trail cameras and tracking tunnels. Our pitfall traps are especially cheap and quick to process as we did not use kill traps and did not identify invertebrates to the species level. Kill traps require a killing agent and specimen jars, increasing the costs per trap. Identifying invertebrates to the species level would increase the processing time and any costs associated with work hours devoted to identifying invertebrates. Tracking tunnels were cheaper than pitfall traps, but they do not collect community composition data, making them a much less effective method. Trail cameras fall behind pitfall traps in cost-effectiveness, as our cameras were both expensive and time-consuming. There are cheaper cameras available, which could make this method more cost-efficient (see Palencia et al. 2022 for a review of camera models and their performance). Still, pitfall traps will likely be more affordable, even with a cheaper camera.
As technology improves, processing data from pitfall traps and trail cameras may become more automated, improving their cost-effectiveness. For pitfall traps, recent studies have created devices that automatically sort, photograph, and identify benthic invertebrate specimens based on the geometric features of the specimen (Ärje et al. 2020; Wührl et al. 2022). Other approaches have used machine learning techniques to identify photographs of invertebrates to the species or sub-family level (Wäldchen and Mäder 2018). For trail cameras, programmes can already classify and identify images of large mammals from trail camera images (Wäldchen and Mäder 2018; Tabak et al. 2019). For invertebrates, however, current methods need to examine pictures of specimens in controlled conditions (e.g., well-lit white backgrounds). They are not quite ready for trail camera images with a complex background where invertebrates are partially shielded from view (Liu et al. 2017).
Some large-bodied alpine invertebrate communities are fairly rare or threatened, and many species are data deficient (Leschen et al. 2012; Sirvid et al. 2012; Trewick et al. 2022). Information on population trends for alpine invertebrates is important for assessing a species’ status and evaluating the effectiveness of conservation efforts and management needs (Monks et al. 2021). Data on population trends are essential in response to an increasing number of threats to alpine species, such as invasive mammalian predators which prey upon large-bodied invertebrates at lower elevations and within the alpine zone (O’Donnell et al. 2017; McAulay et al. 2020; Norbury et al. 2022). To our knowledge, robust, validated monitoring techniques do not exist for alpine invertebrates. Our research highlights promising survey techniques that need to be tested against robust density estimates for key taxonomic groups (Lettink et al. 2011; Carpenter et al. 2016; Monks et al. 2021). If indices generated from pitfall traps are found to be representative of density estimates for large-bodied alpine invertebrates, then pitfall traps could be deployed as a long-term monitoring method in the alpine zone.
Pitfall traps and trail cameras may also be successful monitoring tools for other habitats or biomes that share similar environmental conditions to the New Zealand alpine zone. The alpine zone in New Zealand is characterised by low temperatures, strong wind, and solar radiation (Mark et al. 2000; Buckley et al. 2022). Habitat in alpine areas of New Zealand consists of open tussock grasslands, shrubs and forbs and rock fields (Mark et al. 2000). Temperate grasslands in North America or Asia have similar open grass fields and experience cool temperatures and snow over winter in some regions (Jones and Donnelly 2004). Similarly, boreal forests are associated with low mean temperatures and short growing seasons due to cold, snow-covered winters (Apps et al. 1993). Lastly, subarctic and subantarctic habitats share characteristics with alpine biomes, including low mean temperatures, high winds, prolonged periods of snow and high levels of solar radiation (Smith et al. 2001; Jones and Donnelly 2004). Thus, our results also apply to researchers looking to study ground-dwelling invertebrates in cool, open habitats.
In New Zealand, large-bodied invertebrates are especially important to study due to their vulnerability to introduced predators. However, there are other reasons to study large-bodied invertebrates, which further supports the need for cost-effective ways to monitor them. For example, large invertebrates are easier to see and identify in the field making data collection easier and allowing for more novel methods like trail cameras to be more successful (Gerlach et al. 2013; Hobbs and Brehme 2017). Large-bodied invertebrates can be more charismatic and may be used as flagship species to promote invertebrate conservation. The tiger beetle, for example, has become a global flagship for beetle conservation resulting in studies and conservation management across Europe and North America (New 2010). Stag beetles (Lucanus cervus) have been used to raise awareness around the importance of deadwood habitat in suburban London (New and Samways 2014). In New Zealand, the giant wētā (Anostostomatidae spp.) have been used to increase awareness of the plight of native species faced with predation by introduced predators (New and Samways 2014). Lastly, many large-bodied invertebrates have been used as indicator species (Gerlach et al. 2013) to help researchers understand how invertebrate communities respond to global change. For example Audino et al. (2014) used dung beetles as an indicator of success for tropical forest restoration project. In another example, Bazelet and Samways (2011) found some grasshopper species to be successful indicators of the quality of ecological networks in a mixed system of grasslands and exotic timber plantations. Thus, there are numerous incentives to improve our ability to monitor large-bodied invertebrates across all regions.
Conclusion
Our method comparison suggests that pitfall traps perform well as the primary survey method to monitor large-bodied ground-dwelling invertebrates in a range of habitat types, characteristic of the alpine zone. The low cost of pitfall traps makes this tool accessible for projects with limited budgets, while their simple design makes them easy to scale up to fit the needs of larger projects. That said, the best monitoring method for a given study depends on the study’s objective, the invertebrate groups being targeted and the desired management outcomes. With proven surveying methods, we can better understand large-bodied invertebrates leading to improved conservation outcomes for invertebrates in the alpine zone and other similar regions. Bettering our understanding of invertebrates would contribute to goals of protecting ‘biodiversity’ given that invertebrates represent a large portion of this in New Zealand and across the globe.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson MJ (2014) Permutational multivariate analysis of variance (PERMANOVA). Wiley statsref: statistics reference online 1–15
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biol Invasions 11:1743–1754
Apps M, Kurz W, Luxmoore R et al (1993) Boreal forests and tundra. Water Air Soil Pollut 70:39–53
Ärje J, Melvad C, Jeppesen MR et al (2020) Automatic image-based identification and biomass estimation of invertebrates. Methods Ecol Evol 11:922–931. https://doi.org/10.1111/2041-210X.13428
Audino LD, Louzada J, Comita L (2014) Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol Conserv 169:248–257. https://doi.org/10.1016/j.biocon.2013.11.023
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Ind 11:1259–1269. https://doi.org/10.1016/j.ecolind.2011.01.005
Bertoia A, Monks J, Knox C, Cree A (2021) A nocturnally foraging gecko of the high-latitude alpine zone: Extreme tolerance of cold nights, with cryptic basking by day. J Therm Biol 99:102957
Blackwell GL, Potter MA, McLennan JA (2002) Rodent density indices from tracking tunnels, snap-traps and Fenn traps: do they tell the same story? New Z J Ecol 26:43–51
Bouget C, Brustel H, Brin A, Noblecourt T (2008) Sampling saproxylic beetles with window flight traps: methodological insights. Revue D’écologie
Bowie MH, Allen WJ, McCaw J, van Heugten R (2014) Factors influencing occupancy of modified artificial refuges for monitoring the range-restricted Banks Peninsula tree weta Hemideina ricta (Anostostomatidae). New Z J Ecol 132–138
Brown K, Moller H, Innes J, Alterio N (1996) Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Z J Ecol 271–275
Buchholz S, Möller M (2018) Assessing spider diversity in grasslands–does pitfall trap color matter? J Arachnology 46:376–379
Buchholz S, Corman A-M, Hertenstein F, Schirmel J (2010) Effect of the colour of pitfall traps on their capture efficiency of carabid beetles (Coleoptera: Carabidae), spiders (Araneae) and other arthropods. Eur J Entomol 107:277–280. https://doi.org/10.14411/eje.2010.036
Buckley TR, Hoare RJB, Leschen RAB (2022) Key questions on the evolution and biogeography of New Zealand alpine insects. J Royal Soc New Z 0:1–25. https://doi.org/10.1080/03036758.2022.2130367
Burton AC, Neilson E, Moreira D et al (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52:675–685
Campanaro A, Bardiani M (2012) Walk transects for monitoring of Lucanus cervus in an italian lowland forest. Studia Forestalia Slovenica 137:17–22
Cane J (2021) Global warming, advancing bloom and evidence for pollinator plasticity from long-term bee emergence monitoring. Insects 12:457
Cardoso P, Scharff N, Gaspar C et al (2008) Rapid biodiversity assessment of spiders (Araneae) using semi-quantitative sampling: a case study in a Mediterranean forest. Insect Conserv Divers 1:71–84
Carpenter JK, Monks JM, O’Donnell CFJ (2016) Developing indices of relative abundance for monitoring cave and ground wētā (Orthoptera) in southern beech forest, New Zealand. New Z J Zool 43:149–162
Chinn WGH, Chinn TJH (2020) Tracking the snow line: responses to climate change by New Zealand alpine invertebrates. Antarct Alp Res 52:361–389
Collett RA, Fisher DO (2017) Time-lapse camera trapping as an alternative to pitfall trapping for estimating activity of leaf litter arthropods. Ecol Evol 7:7527–7533
Corti R, Larned ST, Datry T (2013) A comparison of pitfall-trap and quadrat methods for sampling ground-dwelling invertebrates in dry riverbeds. Hydrobiologia 717:13–26. https://doi.org/10.1007/s10750-013-1563-0
Culbertson KA, Garland MS, Walton RK et al (2022) Long-term monitoring indicates shifting fall migration timing in monarch butterflies (Danaus plexippus). Glob Change Biol 28:727–738. https://doi.org/10.1111/gcb.15957
Digweed SC (1995) Digging out the” digging-in effect” of pitfall traps: influences of depletion and disturbance on catches of ground beetles (Coleoptera: Carabidae). Pedobiologia 39:561–576
Doxon ED, Davis CA, Fuhlendorf SD (2011) Comparison of two methods for sampling invertebrates: vacuum and sweep-net sampling. J Field Ornithol 82:60–67
Elliott GP, Kemp J, Russell JC (2018) Estimating population growth rates from tracking tunnels. New Z J Ecol 42:269–272
Engel J, Hertzog L, Tiede J et al (2017) Pitfall trap sampling bias depends on body mass, temperature, and trap number: insights from an individual-based model. Ecosphere 8:e01790
Ernakovich JG, Hopping KA, Berdanier AB et al (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20:3256–3269
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850. https://doi.org/10.1007/s10841-013-9565-9
Gibson S, Penniket S, Cree A (2015) Are viviparous lizards from cool climates ever exclusively nocturnal? Evidence for extensive basking in a New Zealand gecko. Biol J Linn Soc 115:882–895
Gobbi M, Barragán Á, Brambilla M et al (2018) Hand searching versus pitfall trapping: how to assess biodiversity of ground beetles (Coleoptera: Carabidae) in high altitude equatorial Andes? J Insect Conserv 22:533–543. https://doi.org/10.1007/s10841-018-0082-8
Goldson S, Bourdôt G, Brockerhoff E et al (2015) New Zealand pest management: current and future challenges. J Royal Soc New Z 45:31–58
Gurdebeke S, Maelfait J-P (2002) Pitfall trapping in population genetics studies: finding the right “solution. J Arachnology 30:255–261
Hobbs MT, Brehme CS (2017) An improved camera trap for amphibians, reptiles, small mammals, and large invertebrates. PLoS ONE 12:e0185026
Hohbein RR, Conway CJ (2018) Pitfall traps: a review of methods for estimating arthropod abundance. Wildl Soc Bull 42:597–606
Jackson MM, Gergel SE, Martin K (2015) Effects of climate change on habitat availability and configuration for an endemic coastal alpine bird. PLoS ONE 10:e0142110
Jones MB, Donnelly A (2004) Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol 164:423–439
Kammerer M, Tooker JF, Grozinger CM (2020) A long-term dataset on wild bee abundance in Mid-Atlantic United States. Sci Data 7:240. https://doi.org/10.1038/s41597-020-00577-0
Kemp JR, Young L, Mosen C et al (2022) Irruptive dynamics of invasive carnivores and prey populations, and predator control, affect kea survivorship across the Southern Alps. New Z J Zool 0:1–26. https://doi.org/10.1080/03014223.2021.2021249
King CM, Scurr D (2013) Optimizing the ratio of captures to trapping effort in a black rat Rattus rattus control programme in New Zealand. Conserv Evid 95–97
Lang A (2000) The pitfalls of pitfalls: a comparison of pitfall trap catches and absolute density estimates of epigeal invertebrate predators in arable land. J Pest Sci 73:99–106
Lee MS, Comas J, Stefanescu C, Albajes R (2020) The Catalan butterfly monitoring scheme has the capacity to detect effects of modifying agricultural practices. Ecosphere 11:e03004
Leschen RAB, Marris JWM, Emberson RM et al (2012) The conservation status of New Zealand Coleoptera. New Z Entomol 35:91–98. https://doi.org/10.1080/00779962.2012.686311
Lettink M, O’Donnell CFJ, Hoare JM (2011) Accuracy and precision of skink counts from artificial retreats. New Z J Ecol 35:236–246
Lindenmayer DB, Likens GE, Andersen A et al (2012) Value of long-term ecological studies. Austral Ecol 37:745–757
Litt AR, Cord EE, Fulbright TE, Schuster GL (2014) Effects of invasive plants on arthropods. Conserv Biol 28:1532–1549
Liu H, Lee S-H, Chahl JS (2017) A review of recent sensing technologies to detect invertebrates on crops. Precision Agric 18:635–666. https://doi.org/10.1007/s11119-016-9473-6
Luff ML (1975) Some features influencing the efficiency of pitfall traps. Oecologia 345–357
Mark AF, Dickinson KJM, Hofstede RGM (2000) Alpine vegetation, plant distribution, life forms, and environments in a perhumid New Zealand region: oceanic and tropical high mountain affinities. Arct Antarct Alp Res 32:240–254. https://doi.org/10.1080/15230430.2000.12003361
McAulay J, Seddon PJ, Wilson DJ, Monks JM (2020) Stable isotope analysis reveals variable diets of stoats (Mustela erminea) in the alpine zone of New Zealand. New Z J Ecol 44:1–13
Meiners JM, Griswold TL, Carril OM (2019) Decades of native bee biodiversity surveys at Pinnacles National Park highlight the importance of monitoring natural areas over time. PLoS ONE 14:e0207566
Monks JM, O’Donnell CF, Greene TC, Weston KA (2021) Evaluation of counting methods for monitoring populations of a cryptic alpine passerine, the rock wren (Passeriformes, Acanthisittidae, Xenicus gilviventris). PLoS ONE 16:e0247873
Nathan HW, Clout MN, Murphy EC, MacKay JW (2013) Strategies for detection of house mice on a recently invaded island. New Z J Ecol 26–32
New TR (2010) Beetle conservation. In: New TR (ed) Beetles in conservation. Springer, Netherlands, p 94
New TR, Samways MJ (2014) Insect conservation in the southern temperate zones: an overview. Austral Entomol 53:26–31
Norbury G, Wilson DJ, Clarke D et al (2022) Density-impact functions for invasive house mouse (Mus musculus) effects on indigenous lizards and invertebrates. Biol Invasions 1–15
O’Brien TG, Kinnaird MF (2008) A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv Int 18:S144–S162
O’Donnell CF, Weston KA, Monks JM (2017) Impacts of introduced mammalian predators on New Zealand’s alpine fauna. New Z J Ecol 41:1–22
Oksanen J, Blanchet FG, Friendly M et al (2020) vegan: Community Ecology Package
Palencia P, Vicente J, Soriguer RC, Acevedo P (2022) Towards a best-practices guide for camera trapping: assessing differences among camera trap models and settings under field conditions. J Zool 316:197–208. https://doi.org/10.1111/jzo.12945
Paler K, Monks A, Leschen RA, Ward DF (2021) Determining species diversity and functional traits of beetles for monitoring the effects of environmental change in the New Zealand alpine zone. Ecol Ind 121:107100
Pekár S (2002) Differential effects of formaldehyde concentration and detergent on the catching efficiency of surface active arthropods by pitfall traps. Pedobiologia 46:539–547
Perner J, Schueler S (2004) Estimating the density of ground-dwelling arthropods with pitfall traps using a nested-cross array. J Anim Ecol 469–477
Popic TJ, Davila YC, Wardle GM (2013) Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps. PLoS ONE 8
Portman ZM, Bruninga-Socolar B, Cariveau DP (2020) The state of bee monitoring in the United States: a call to refocus away from bowl traps and towards more effective methods. Ann Entomol Soc Am 113:337–342
Potter TI, Greenville AC, Dickman CR (2021) Night of the hunter: using cameras to quantify nocturnal activity in desert spiders. PeerJ 9:e10684
Riley KN, Browne RA, Erwin TL (2016) Results from two sampling techniques for carabid beetles (Coleoptera: Carabidae) in temporarily flooded and terra firme rainforest of western Amazonia. Stud Neotropical Fauna Environ 51:78–95
RStudio Team, RStudio (2020) RStudio: Integrated Development Environment for R. PBC., Boston, MA
Ruiz-Lupión D, Pascual J, Melguizo-Ruiz N et al (2019) New Litter Trap Devices Outperform Pitfall Traps for studying arthropod activity. Insects 10:147. https://doi.org/10.3390/insects10050147
Ruscoe WA, Sweetapple PJ, Perry M, Duncan RP (2013) Effects of spatially extensive control of invasive rats on abundance of native invertebrates in mainland New Zealand forests. Conserv Biol 27:74–82
Schmidt O, Schmidt S, Häuser CL et al (2019) Using Malaise traps for collecting Lepidoptera (Insecta), with notes on the preparation of Macrolepidoptera from ethanol. Biodiversity data journal
Schneider E, Steinwandter M, Seeber J (2019) A comparison of Alpine soil macro-invertebrate communities from european larch and swiss pine forests in the LTSER area “Val Mazia/Matschertal”, South Tyrol. Gredleriana 19:217–228
Schori JC, Steeves TE, Murray TJ (2020) Designing monitoring protocols to measure population trends of threatened insects: a case study of the cryptic, flightless grasshopper Brachaspis robustus. PLoS ONE 15:e0238636. https://doi.org/10.1371/journal.pone.0238636
Sirvid PJ, Vink CJ, Wakelin MD et al (2012) The conservation status of New Zealand Araneae. New Z Entomol 35:85–90. https://doi.org/10.1080/00779962.2012.686310
Skvarla M, Larson J, Dowling A (2014) Pitfalls and preservatives: a review. J Entomol Soc Ont 145
Smith VR, Steenkamp M, Gremmen NJM (2001) Terrestrial habitats on sub-antarctic Marion Island: their vegetation, edaphic attributes, distribution and response to climate change. South Afr J Bot 67:641–654. https://doi.org/10.1016/S0254-6299(15)31195-9
Smith D, Jamieson I, Peach R (2005) Importance of ground weta (Hemiandrus spp.) in stoat (Mustela erminea) diet in small montane valleys and alpine grasslands. New Z J Ecol 207–214
Standen V (2000) The adequacy of collecting techniques for estimating species richness of grassland invertebrates. J Appl Ecol 37:884–893
Stringer I, Watts C, Thornburrow D et al (2014) Saved from extinction? Establishment and dispersal of Mercury Islands tusked weta, Motuweta isolata, following translocation onto mammal-free islands. J Insect Conserv 18:203–214
Tabak MA, Norouzzadeh MS, Wolfson DW et al (2019) Machine learning to classify animal species in camera trap images: applications in ecology. Methods Ecol Evol 10:585–590. https://doi.org/10.1111/2041-210X.13120
Trewick S, Danilo H, Mary M-R et al (2022) Conservation status of New Zealand Orthoptera, 2022. Department of Conservation, Wellington
Uetz GW, Unzicker JD (1975) Pitfall trapping in ecological studies of wandering spiders. J Arachnology 101–111
van Swaay CA, Nowicki P, Settele J, Van Strien AJ (2008) Butterfly monitoring in Europe: methods, applications and perspectives. Biodivers Conserv 17:3455–3469
Wäldchen J, Mäder P (2018) Machine learning for image based species identification. PLoS ONE 9:2216–2225. https://doi.org/10.1111/2041-210X.13075
Watts C, Thornburrow D (2011) Habitat use, behavior and movement patterns of a threatened New Zealand giant weta, Deinacrida heteracantha (Anostostomatidae: Orthoptera). J Orthoptera Res 127–135
Watts C, Thornburrow D, Green C, Agnew W (2008) Tracking tunnels: a novel method for detecting a threatened New Zealand giant weta (Orthoptera: Anostostomatidae). New Z J Ecol 92–97
Watts C, Stringer I, Thornburrow D, MacKenzie D (2011) Are footprint tracking tunnels suitable for monitoring giant weta (Orthoptera: Anostostomatidae)? Abundance, distribution and movement in relation to tracking rates. J Insect Conserv 15:433–443. https://doi.org/10.1007/s10841-010-9321-3
Wepprich T, Adrion JR, Ries L et al (2019) Butterfly abundance declines over 20 years of systematic monitoring in Ohio, USA. PLoS ONE 14:e0216270
Wilson DJ, Lee WG (2010) Primary and secondary resource pulses in an alpine ecosystem: snow tussock grass (Chionochloa spp) flowering and house mouse (Mus musculus) populations in New Zealand. Wildl Res 37:89–103
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royal Stat Society: Ser B (Statistical Methodology) 73:3–36
Woodcock BA (2005) Pitfall Trapping in Ecological Studies. Insect sampling in Forest Ecosystems. John Wiley & Sons, Ltd, pp 37–57
Wührl L, Pylatiuk C, Giersch M et al (2022) DiversityScanner: robotic handling of small invertebrates with machine learning methods. Mol Ecol Resour 22:1626–1638. https://doi.org/10.1111/1755-0998.13567
Wynne JJ, Howarth FG, Sommer S, Dickson BG (2019) Fifty years of cave arthropod sampling: techniques and best practices. KIP Articles
Zaller J, Kerschbaumer G, Rizzoli R et al (2015) Monitoring arthropods in protected grasslands: comparing pitfall trapping, quadrat sampling and video monitoring. Web Ecol 15:15
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions for this research was provided by the New Zealand Department of Conservation and the University of Otago. The authors have no competing interests to declare. Our research followed consultation with the Ngāi Tahu Research Consultation Committee of the University of Otago. This research was conducted as part of DOC’s alpine research programme.
Author information
Authors and Affiliations
Contributions
Aaron Bertoia: Conceptualization (equal), Methodology (supporting), Formal Analysis (lead), Investigation (lead), Visualization (lead), Preparation (lead), Validation (equal), Writing—Original Draft (lead), Writing—Review & Editing (equal). Tarra Murray: Conceptualization (equal), Methodology (equal), Writing—Review & Editing (equal), Supervision (supporting). Bruce Robertson: Writing—Review & Editing (equal), Methodology (supporting), Supervision (supporting). Jo Monks: Conceptualization (equal), Methodology (equal), Resources (lead), Supervision (lead), Validation (equal), Writing—Review & Editing (equal), Funding Acquisition (lead).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bertoia, A., Murray, T., Robertson, B.C. et al. Pitfall trapping outperforms other methods for surveying ground-dwelling large-bodied alpine invertebrates. J Insect Conserv 27, 679–692 (2023). https://doi.org/10.1007/s10841-023-00498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00498-4