Abstract
Background
Pulmonary vein isolation (PVI) has become the cornerstone treatment of atrial fibrillation (AF). While in cryoablation cell damage is caused by thermal effects, lately, pulsed field ablation (PFA) has been established as a novel non-thermal tissue-specific ablation modality for PVI. However, data comparing outcomes of patients undergoing either PFA or cryoballoon ablation (CBA) for primary PVI are sparse.
Methods
Consecutive patients with AF undergoing PVI by either CBA or PFA were included in the analysis. The primary outcome was the time to AF/AT recurrence. For secondary outcomes, clinical and periprocedural parameters were compared.
Results
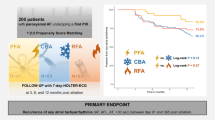
In total, outcomes of 141 AF patients treated by PFA (94 patients) or CBA (47 patients) were compared. After 365 days, 70% of patients in the PFA group and 61% of patients in the CBA group were free from AF/AT (HR 1.35, 95% CI 0.60–3.00; p = 0.470). No deaths occurred. While symptoms alleviated in both groups, only after PFA, we observed significant improvement of left atrial volume index (PFA group baseline: 40 [31;62] ml/m2, PFA group follow-up: 35 [29;49] ml/m2; p = 0.015), NT-pro BNP levels (PFA group baseline: 1106 ± 2479 pg/ml, PFA group follow-up: 1033 ± 1742 pg/ml; p = 0.048), and left ventricular ejection fraction (LVEF) (PFA group baseline: 55 [48;60] %, PFA group follow-up: 58 [54;63] %; p = 0.006). PVI by PFA was the only independent predictor of LVEF improvement.
Conclusion
In our study, we show that CBA and PFA for PVI are of similar efficacy when it comes to AF recurrence. However, our findings suggest that PFA rather than CBA might induce left atrial reverse remodeling thereby contributing to left ventricular systolic function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) for atrial fibrillation (AF) is a hallmark of AF treatment and can be achieved by a variety of methodical approaches. The efficacy of conventional ablation techniques improved over the past years, but freedom from atrial arrhythmia is still between 71 and 86% in patients with paroxysmal AF after 1 year [1,2,3]. Therefore, alternative ablation techniques are needed to improve long-term ablation success. While thermal ablation modalities, such as cryoballoon ablation (CBA) or radiofrequency ablation (RFA), have been established as cornerstone therapy over the past years, lately, pulsed field ablation (PFA) has been introduced as a novel non-thermal tissue-specific ablation modality for PVI. By application of ultra-rapid electrical pulses, PFA generates an electrical field disrupting cell membranes by creating nanoscale pores, ultimately leading to cell death. Remarkably, at a defined energy threshold, electroporation has been shown to be nearly myocardium-specific, thereby minimizing the potential collateral damage to non-target tissue, while allowing effective ablation of cardiomyocytes in a defined area [4]. However, data comparing periprocedural and rhythm outcomes of patients undergoing pulmonary vein isolation for atrial fibrillation by either cryoballoon ablation or pulsed field ablation are limited. Additionally, information regarding the effect of PFA for PVI on atrial and ventricular structure and function are sparse, especially in comparison to CBA.
2 Methods
2.1 Study population
We conducted a case-control study taking into account patients treated at the Department of Cardiology and Angiology at Essen University Medical Center, Essen, Germany, with paroxysmal or persistent atrial fibrillation with an indication for first-do PVI according to the current ESC guideline [5]. Consecutive patients undergoing either cryoballoon ablation (POLARx™, Boston Scientific, Massachusetts, USA) or pulsed field ablation (FARAWAVE™, Boston Scientific, Massachusetts, USA), were included. The ablation modality was chosen at the discretion of the interventionalist. Patients with decompensated heart failure, cardiogenic shock, or patients scheduled for repeat ablation were not eligible. The study complies with the Declaration of Helsinki and was approved by the local ethics committee (University of Duisburg-Essen).
2.2 Preprocedural management and ablation procedure
Preprocedural management was performed as previously described [6, 7]. In brief, left atrial thrombus was ruled out by transesophageal echocardiography before the procedure in all patients prior to PVI. Otherwise, no preprocedural imaging was routinely acquired. Vitamin K antagonists were administered uninterruptedly with a target INR of 2.0–2.5 at the time of procedure. Patients treated with non-VKA oral anticoagulants were advised to hold their anticoagulant <24 h prior to the ablation procedure. All procedures were performed under deep sedation with continuous heparin administration during the procedure by activated clotting time goal of 300-350ms. In all patients a diagnostic catheter was placed in the coronary sinus, and an additional quadripolar catheter in the right ventricle, respectively for phrenic nerve capture if required. The respective ablation catheter was advanced to the left atrium after a single transseptal puncture. After successful transseptal puncture and introduction of the respective steerable sheath into the left atrium pulmonary vein (PV), angiography was performed to capture PV anatomy. In the PFA group, a 12-French over-the-wire PFA ablation catheter (FARAWAVE™, Boston Scientific, Massachusetts, USA) was used in either a flower or basket configuration delivering the energy in a set of microsecond-scale biphasic pulses of 1800–2000 V in bipolar approach across all electrodes. Each application was made of five pulse packets delivered over a few seconds. Applications were repeated eight times per vein, four times in flower, four times in basket configuration, with repositioning and/or rotation of the catheter every two applications. Before energy delivery the ablation catheter was positioned at the proximal border of the PV ostium according to the PV angiography to ensure circumferential PV ostial and antral coverage as good as possible. The procedural endpoint of complete PVI was confirmed by bidirectional conduction block after PFA applications. In patients undergoing cryoballoon ablation, PVI was performed using a 28-mm cryoballoon catheter (POLARx™, Boston Scientific, Massachusetts, USA) under fluoroscopic guidance. To achieve PV occlusion, the CB was inflated proximal to the PV ostium and complete occlusion of the PV ostium was verified by injections of contrast media. The standard freeze-cycle duration was 180 s. During PVI of the septal PVs, continuous phrenic nerve (PN) pacing was performed using a diagnostic catheter placed within the superior vena cava. Pacing was set at maximum output and pulse width. PN capture was also monitored by the tactile feedback of diaphragmatic contraction. After PVI, entrance and exit block were confirmed by either placing the PolarMapTM catheter or the FARAWAVETM catheter within the PVs and pacing maneuvers were performed as recommended. Additionally, pre- and post-ablation three-dimensional ultra-high-density mapping was conducted at the discretion of the interventionalist in both groups.
2.3 Postprocedural management and clinical follow-up
Echocardiography was performed in every patient immediately after the procedure and before hospital discharge to rule out pericardial effusion or pericardial tamponade. All patients underwent a neurological examination directly after the procedure to detect potential focal neurological deficits. Oral anticoagulation was resumed in the evening after the intervention in the absence of groin complications. Anti-arrhythmic drugs were discontinued immediately after the procedure. At the first day after the procedure a 12-lead ECG was written to determine the current rhythm. Patients were scheduled for outpatient clinic visits including clinical assessment, transthoracic echocardiography, and 7-day Holter monitoring at 3 and 6 months after the procedure and thereafter every 6 months. Any documented sustained atrial tachyarrhythmia on 12-lead ECG or any tachyarrhythmia of 30 s or longer on Holter ECG was counted as AF recurrence. All patients were off anti-arrhythmic drugs. A blanking period of 90 days was applied.
2.4 Primary and secondary outcomes
The primary outcome was the time to AF/AT recurrence. Secondary outcomes were procedural parameters and periprocedural complications. Furthermore, clinical parameters at baseline and at the end of follow-up were compared between both groups. Start of the follow-up period was defined as the day of the ablation procedure.
2.5 Statistical analysis
The significance of differences of numeric values was calculated by t test if normal distribution with equal variance was given. Numeric variables that were not normally distributed were analyzed by Mann–Whitney rank sum test and described as median and first to third interquartile range. Categorical variables were described as absolute and relative values and analyzed by χ2 test or Fisher’s exact test, as appropriate. Kaplan–Meier analysis was used to assess the time to event and groups were compared using the Cox proportional hazard model. For identification of independent predictors of LVEF improvement, as defined by any numeric LVEF improvement in the echocardiographic examination at the end of follow-up, baseline characteristics were analyzed by univariate logistic regression. Parameters with a p ≤ 0.1 were further tested for independency by multivariate logistic regression. A p < .05 was considered to be statistically significant. Statistical assessment was performed by SPSS Statistics 27 software (Version 2020; IBM).
3 Results
3.1 Study population
A total of 141 consecutive patients, 47 patients treated by CBA and 94 by PFA, were included in our study. Patients had a median age of 63 years and were predominantly male (66%). Type of atrial fibrillation (AF) was equally distributed between ablation groups (p = 0.575). Patients treated by PFA were significantly more often diagnosed with arterial hypertension (CBA group: 26 out of 47 patients, PFA group: 73 out of 94 patients; p = 0.006), while diabetes mellitus was observed more often in the CBA group (CBA group: 15 out of 47 patients, PFA group: 16 out of 94 patients; p = 0.044). Otherwise, there were no significant differences in baseline characteristics between both groups as listed in Table 1.
3.2 Arrhythmia recurrence
Arrhythmia recurrence was compared between AF patients undergoing either CBA or PFA for PVI. There was no significant difference after 365 days between the groups (HR 1.35, 95% CI 0.60–3.00; p = 0.470; Fig. 1). In total, after PFA, 70% had no atrial fibrillation/atrial tachycardia (AF/AT) recurrence after 365 days compared to 61% after CBA. For patients with paroxysmal AF, 87% of patients from the PFA group and 83% of patients from the CBA group; and for persistent AF, 57% of patients from the PFA group and 58% of patients from the CBA group were still free from AF. With regard to long-standing persistent AF, 27% of patients from the PFA group and 20% of patients from the CBA group had no AF/AT recurrence. No deaths occurred in both groups.
3.3 Procedural parameters and periprocedural complications
Assessment of procedural parameters showed no significant difference for total procedure time and fluoroscopic time between both groups. However, the amount of contrast dye used during the procedure (CBA group, 154 ± 82 ml; PFA group, 99 ± 66 ml; p < 0.001) was significantly lower in the PFA group. There were no significant differences in periprocedural complications (CBA group, 1 patient; PFA group, 4 patients; p = 0.664). All patients had full recovery. Procedural parameters are detailed in Table 2.
3.4 Clinical parameters at baseline and at the end of follow-up
To further evaluate the effect of both ablation modalities on clinical outcome, AF patients’ symptoms as measured by NYHA- and EHRA-class, as well as serum NT-pro BNP levels, left ventricular ejection fraction (LVEF), and left atrial volume index (LAVI), were assessed at baseline and at the end of follow-up.
Median EHRA-class (CBA group baseline (CBA-bl): 3 [2; 3], CBA group follow-up (CBL-fu): 0 [0; 2]; p < 0.001; PFA group baseline (PFA-bl): 3 [2; 3], PFA group follow-up (PFA-fu): 1 [0;2]; p < 0.001) and median NYHA-class (CBA-bl: 2 [2; 3], CBA-fu: 2 [1; 2]; p = 0.002; PFA-bl: 2 [2; 3], PFA-fu: 1 [1; 2]; p < 0.001) improved significantly in both groups. Remarkably, we observed a significant decrease of NT-pro BNP serum levels (PFA-bl: 1106 ± 2479 pg/ml, PFA-fu: 1033 ± 1742 pg/ml; p = 0.048) and left atrial volume index (PFA-bl: 40 [31; 62] ml/m2, PFA-fu: 35 [29; 49] ml/m2; p = 0.015) in patients treated with PFA. LVEF increased in this population (PFA-bl: 55 [48; 60] %, PFA-fu: 58 [54; 63] %; p = 0.006). This was only seen in patients undergoing PVI by PFA, while there were no significant differences in patients treated by CBA.
There were no significant differences between the groups both at baseline and at the end of follow-up. Clinical parameters are shown in Table 3.
3.5 Identification of predictors of left ventricular ejection fraction improvement
To identify independent predictors of LVEF improvement, we performed uni- as well as multivariate logistic regression analysis. In univariate logistic regression analysis, we identified sex and PVI by PFA as potential predictors of LVEF improvement. However, in the multivariate analysis only PVI by PFA remained independently and significantly associated with improvement of LVEF (HR 3.70, 95% CI 1.19–11.49; p = 0.024; Table 4).
4 Discussion
In this study, we compared outcomes of patients undergoing either cryoballoon ablation (CBA) or pulsed field ablation (PFA) for pulmonary vein isolation (PVI) in atrial fibrillation (AF) patients.
We found that after 365 days, there was no significant difference in AF/AT recurrence between both groups. The 1-year success rates between both groups were similar for patients with paroxysmal AF (PFA-group: 87%; CBA-group: 83%), persistent AF (PFA-group: 57%; CBA-group: 58%), and long-standing persistent AF (PFA-group: 27%; CBA-group: 20%). Additionally, there was no significant difference in periprocedural complications. The amount of contrast dye used was lower in PFA patients. There was no difference in total procedure time. Compared to baseline, AF patients undergoing PFA showed decreased NT-pro BNP levels, a lower left atrial volume index, and increased left ventricular ejection fraction at the end of follow-up, whereas there were no differences for CBA patients.
4.1 Rhythm outcome
PFA has been introduced as a novel non-thermal and tissue-specific ablation modality achieving PVI by application of rapidly alternating high electrical fields to atrial tissue, thereby inducing nanopores followed by cell death [4]. Current data from the MANIFEST-PF registry and the PULSED AF Pivotal trial showed comparable efficacy rates after 12 months as compared to thermal ablation approaches [8,9,10]. While 78% of patients included in the MANIFEST-PF registry were free from atrial arrhythmia after 365 days, in the PULSED AF Pivotal trial, 66% of patients with paroxysmal AF and 55% of patients with persistent AF were free from any atrial arrhythmia after 1 year [8, 9]. Urbanek et al. were the first to show that single-shot ablation for PVI by PFA is of similar efficacy and safety as compared to CBA. They found that after 1-year, approximately 83% of patients with paroxysmal AF who underwent PFA and 80% of patients who underwent CBA were still free from AF. For patients with persistent AF 1-year success rates were 67% for PFA and 71% for CBA, respectively [11]. The ADVENT-trial, which compared PFA with conventional thermal ablation in paroxysmal AF patients only, reported a 1-year treatment success in 73% of patients treated by PFA and in 71% after thermal ablation [12]. Schipper et al. describe freedom from atrial arrhythmia in 74% of patients who received PFA and in 72% of patients treated by CBA after 365 days [13]. In our study, we observed freedom from AF in 70% of patients in the PFA group and 61% of patients in the CBA group at the end of follow-up. If analyzed by AF type, 87% of paroxysmal AF patients treated by PFA and 83% of patients who underwent CBA were still free from any atrial arrhythmia after 365 days, while freedom from AF in patients with persistent AF was 57% in the PFA group and 58% in the CBA group. This was even lower in patients with long-standing persistent AF (PFA-group: 27%; CBA-group: 20%). Consequently, overall our data are in line with the previously mentioned studies and substantiate that CBA and PFA for PVI appear to be of similar efficacy in the treatment of AF [8,9,10,11]. Additionally, we observed that AF/AT recurrence after PVI is less likely in patients with paroxysmal AF than in patients with persistent and long-standing persistent AF. This underscores that early rhythm control is crucial for long-term maintenance of sinus rhythm [14].
4.2 Procedural parameters
An analysis of procedural parameters showed that the amount of contrast dye used was significantly lower in the PFA group, whereas we did not observe differences regarding the total procedure time. In comparison to the study of Urbanek et al., the total procedure time was longer in our patient population [11]. Supposedly, this is attributable to the use of 3-dimensional ultra-high-density mapping before and after the ablation procedure, which was conducted at the discretion of the interventionalist. That might also have leveled a potential difference in procedure time between both groups. Furthermore, we observed that PFA was associated with a lower amount of contrast dyes used, which is not surprising since PFA does not rely on confirming pulmonary vein occlusion before ablation by injection of contrast dye. Consequently, PFA for PVI might be more suitable for patients with known chronic kidney injury compared to CBA since the exposition to iodinated contrast dye is lower [15]. As for periprocedural complications, our data are comparable with the adverse event rates reported by Schipper et al. and Urbanek et al. [11, 13].
4.3 Clinical outcomes
To evaluate the impact of PFA and CBA for PVI in AF patients on clinical outcomes, symptoms related to arrhythmia as measured by EHRA-class and symptoms related to heart failure as measured by NYHA-class were assessed at baseline and at the end of follow-up. Additionally, NT-pro BNP levels, left atrial volume index (LAVI), and left ventricular ejection fraction (LVEF) were compared to detect potential effects on cardiac function.
We found that symptoms as measured by NYHA-class and EHRA-class ameliorated significantly after PVI at the end of follow-up, which has previously been shown in other studies [16, 17]. This was the same for AF patients who underwent either PFA or CBA for PVI. There was no significant difference between both groups.
Furthermore, the analysis of LAVI showed that at the end of follow-up in the PFA group there were signs of left atrial structural reverse-remodeling, while there was no difference in the CBA group. It is known that atrial remodeling is pivotal for the occurrence and development of AF, and it has been shown that PVI for AF can induce reverse-remodeling, especially in paroxysmal AF. For instance, Wang et al. demonstrated that both radiofrequency ablation and CBA are associated with atrial reverse-remodeling and that, interestingly, CBA even might outperform RFA in this context [18]. Our results suggest that PFA might even be superior to CBA regarding left atrial reverse remodeling. This might be related to the tissue-specificity of PFA consequently reducing the collateral damage inflicted on the left atrium, as compared to CBA [19].
Remarkably, comparison of LVEF revealed that left ventricular systolic function improved in the PFA group but not in the CBA group. Previous studies have shown that atrial reverse-remodeling and improvement of LVEF after restoration of sinus rhythm can be expected to occur after successful PVI for AF, even in patients with normal LVEF [20]. However, the mechanism is only poorly understood. One possible explanation is that left atrial reverse-remodeling improves left ventricular filling [20,21,22]. Since multivariate regression analysis identified PFA ablation as the only variable significantly and independently associated with LVEF improvement, we hypothesize that left atrial reverse-remodeling improves left ventricular filling thereby contributing to left ventricular systolic function. The finding of left atrial reverse-remodeling and the idea of improved cardiac function is substantiated by the significant decrease of NT-pro BNP levels at the end of follow-up only in the PFA group. However, further studies are warranted to verify this hypothesis.
5 Limitations
As this is a single center case control study, comparing the effects of CBA and PFA for PVI in AF patients on AF-freedom and periprocedural efficacy and safety, it inherently has limitations. Due to the novelty of the PFA system, operators had no previous experience using this technology, which might affect the procedural parameters and consequently, a learning curve must be considered. Moreover, since this is a study from a single center, only a limited number of patients could be included. Consequently, our results must be interpreted as hypothesis generating. Multicenter studies are of the essence to verify our results.
6 Conclusion
In this study, we compared cryoballoon ablation (CBA) and pulsed field ablation (PFA) for pulmonary vein isolation (PVI) in atrial fibrillation (AF) regarding rhythm outcomes, procedural parameters, and functional and clinical outcomes. While CBA and PFA for PVI are of similar efficacy when it comes to AF recurrence, PFA was associated with a lower amount of contrast dye used during the intervention. Additionally, our results indicate that PFA rather than CBA might induce left atrial reverse remodeling thereby contributing to left ventricular systolic function.
References
Chun KRJ, Okumura K, Scazzuso F, Keun On Y, Kueffer FJ, Braegelmann KM, et al. Safety and efficacy of cryoballoon ablation for the treatment of paroxysmal and persistent AF in a real-world global setting: results from the Cryo AF Global Registry. J Arrhythm. 2021;37(2):356–67. https://doi.org/10.1002/joa3.12504.
Creta A, Kanthasamy V, Schilling RJ, Rosengarten J, Khan F, Honarbakhsh S, et al. First experience of POLARx™ versus Arctic Front Advance™: an early technology comparison. J Cardiovasc Electrophysiol. 2021;32(4):925–30. https://doi.org/10.1111/jce.14951.
Yap S-C, Anic A, Breskovic T, Haas A, Bhagwandien RE, Jurisic Z, et al. Correction to: comparison of the 1-year clinical outcome of a novel cryoballoon to an established cryoballoon technology. J Interv Card Electrophysiol. 2022;64(3):565–5. https://doi.org/10.1007/s10840-022-01300-2.
Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74(3):315–26. https://doi.org/10.1016/j.jacc.2019.04.021.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612.
Kupusovic J, Kessler L, Bruns F, Bohnen J-E, Nekolla SG, Weber MM, et al. Visualization of fibroblast activation using 68Ga-FAPI PET/CT after pulmonary vein isolation with pulsed field compared with cryoballoon ablation. J Nucl Cardiol. 2023;30(5):2018–28. https://doi.org/10.1007/s12350-023-03220-8.
Wakili R, Siebermair J, Fichtner S, Sinner MF, Klocker E, Olesch L, et al. One-year clinical outcome after ablation with a novel multipolar irrigated ablation catheter for treatment of atrial fibrillation: potential implications for clinical use. Europace. 2016;18(8):1170–8. https://doi.org/10.1093/europace/euv349.
Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF Registry. Circulation. 2023;148(1):35–46. https://doi.org/10.1161/CIRCULATIONAHA.123.064959.
Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023;147(19):1422–32. https://doi.org/10.1161/CIRCULATIONAHA.123.063988.
Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45. https://doi.org/10.1056/NEJMoa1602014.
Urbanek L, Bordignon S, Schaack D, Chen S, Tohoku S, Efe TH, et al. Pulsed field versus cryoballoon pulmonary vein isolation for atrial fibrillation: efficacy, safety, and long-term follow-up in a 400-patient cohort. Circulation. 2023; https://doi.org/10.1161/CIRCEP.123.011920.
Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023; https://doi.org/10.1056/NEJMoa2307291.
Schipper J, Steven D, Lüker J, Wörmann J, van den Bruck J, Filipovic K, et al. Comparison of pulsed field ablation and cryoballoon ablation for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2023; https://doi.org/10.1111/jce.16056.
Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–16. https://doi.org/10.1056/NEJMoa2019422.
Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146–55. https://doi.org/10.1056/NEJMra1805256.
Rattka M, Kühberger A, Pott A, Stephan T, Weinmann K, Baumhardt M, et al. Catheter ablation for atrial fibrillation in HFpEF patients—a propensity-score-matched analysis. J Cardiovasc Electrophysiol. 2021;32(9):2357–67. https://doi.org/10.1111/jce.15200.
Rattka M, Pott A, Kühberger A, Weinmann K, Scharnbeck D, Stephan T, et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. EP Europace. 2020;22(9):1328–36. https://doi.org/10.1093/europace/euaa101.
Wang X, Song B, Qiu C, Han Z, Wang X, Lu W, et al. The effect of left atrial remodeling after cryoballoon ablation and radiofrequency ablation for paroxysmal atrial fibrillation. Clin Cardiol. 2021;44(1):78–84. https://doi.org/10.1002/clc.23507.
Sohns C, Fink T, Braun M, Sciacca V, Piran M, Khalaph M, et al. Lesion formation following pulsed field ablation for pulmonary vein and posterior wall isolation. Pacing Clin Electrophysiol. 2023;46(7):714–6. https://doi.org/10.1111/pace.14727.
Kriatselis C, Unruh T, Kaufmann J, Gerds-Li J-H, Kelle S, Gebker R, et al. Long-term left atrial remodeling after ablation of persistent atrial fibrillation: 7-year follow-up by cardiovascular magnetic resonance imaging. J Interv Card Electrophysiol. 2020;58(1):21–7. https://doi.org/10.1007/s10840-019-00584-1.
Inciardi RM, Bonelli A, Biering-Sorensen T, Cameli M, Pagnesi M, Lombardi CM, et al. Left atrial disease and left atrial reverse remodelling across different stages of heart failure development and progression: a new target for prevention and treatment. Eur J Heart Fail. 2022;24(6):959–75. https://doi.org/10.1002/ejhf.2562.
dos Santos SN, Henz BD, Zanatta AR, Barreto JR, Loureiro KB, Novakoski C, et al. Impact of atrial fibrillation ablation on left ventricular filling pressure and left atrial remodeling. Arq Bras Cardiol. 2014;103(6):485–92. https://doi.org/10.5935/abc.20140152.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work is supported by the Open Access Publication Fund of the University of Duisburg Essen.
Author information
Authors and Affiliations
Contributions
MR and SM had the idea for and designed the study and had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis. EM, DV, IR, JB, CK, LY, and CJ collected the data. MR performed the statistical analysis. MR, CJ, and SM mainly wrote the manuscript with support from IR, JB, RW, JS, and TR. SM and MR were mainly responsible for the interpretation of the data, and SM and TR supervised the project. All the authors provided critical feedback and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rattka, M., Mavrakis, E., Vlachopoulou, D. et al. Pulsed field ablation and cryoballoon ablation for pulmonary vein isolation: insights on efficacy, safety and cardiac function. J Interv Card Electrophysiol (2024). https://doi.org/10.1007/s10840-024-01748-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-024-01748-4