Abstract
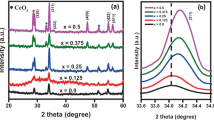
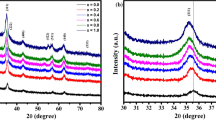
Lithium and iron co-doped cadmium oxide Cd0.9(Li1-xFex)0.1O (x = 0.1, 0.3, 0.5, 0.7) with NaCl structure has been synthesized using formate of the composition Cd0.9(Li1-xFex)0.1(HCOO)2·2H2O as a precursor. The NMR spectroscopy results demonstrate that the structure of lithium-doped cadmium oxide appears to have impurity centers only of one type. All the synthesized samples show a metal-like conductivity as indicated by the growth of their electrical resistance with temperature increasing in the interval 78–330 K. The study of the magnetic properties of the Cd0.9(Li1-xFex)0.1O samples at 5 and 300 K revealed that they are ferromagnets, whose saturation magnetization increases with the iron concentration both at low and room temperature reaching the maximal values in the samples with a Li and Fe concentration of 3 and 7 at.%, respectively. An enhancement of the iron concentration in Cd0.9(Li1-xFex)0.1O from x = 0.5 to x = 0.7 leads to an abrupt growth of the magnetization from 0.30 to 1.94 emu/g at 5 K and from 0.16 to 1.03 emu/g at 300 K. Iron doping with a simultaneous reduction of the lithium concentration also results in an increase of the band gap. The properties of these compounds are explained on the basis of first-principles calculations of their band structure.
















Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
F.A. Benko, F.P. Koffyberg, Quantum efficiency and optical transitions of CdO photoanodes. Solid State Commun. 57, 901 (1986). https://doi.org/10.1016/0038-1098(86)90920-8
S. Ghotekar, A review on plant extract mediated biogenic synthesis of CdO nanoparticles and their recent applications. Asian J. Green Chem. 3, 187 (2019). https://doi.org/10.22034/ajgc.2018.140313.1084
A. Tadjarodi, M. Imani, A novel nanostructure of cadmium oxide synthesized by mechanochemical method. Mater. Res. Bull. 46, 1949 (2011). https://doi.org/10.1016/j.materresbull.2011.07.016
A. Tadjarodi, M. Imani, H. Ke, Application of a facile solid-state process to synthesize the CdO spherical nanoparticles. Intern. Nano Lett. 3, 43 (2013). https://doi.org/10.1186/2228-5326-3-43
N. Shanmugam, B. Saravanan, R. Reagan, N. Kannadasan, K. Sathishkumar, S. Cholan, Effect of thermal annealing on the Cd(OH)2 and preparation of CdO nanocrystals. Mod. Chem. Appl. 2, 1000124 (2014). https://doi.org/10.4172/2329-6798.1000124
Q. Lu, S.F. Wang, L.J. Li, J.L. Wang, S.Y. Dai, W. Yu, G.S. Fu, Electrical and thermal transport properties of CdO ceramics. Sci. China-Phys. Mech. Astron. 57, 1644 (2014). https://doi.org/10.1007/s11433-014-5405-5
X. Zhang, H. Li, J. Wang, Effect of sintering temperature on thermoelectric properties of CdO ceramics. J. Adv. Ceram. 4, 226 (2015). https://doi.org/10.1007/s40145-015-0153-1
R. Liu, L. Gao, L. Li, X. Zha, J. Wang, G. Fu, S. Wang, Enhanced high-temperature thermoelectric performance of CdO ceramics with randomly distributed micropores. J. Am. Ceram. Soc. 100, 3239 (2017). https://doi.org/10.1111/jace.14849
K. Anandhan, R.T. Kumar, Synthesis, FTIR, UV–Vis and Photoluminescence characterizations of triethanolamine passivated CdO nanostructures. Spectrochim. Acta A. 149, 476 (2015). https://doi.org/10.1016/j.saa.2015.04.035
N. Thovhogi, E. Park, E. Manikandan, M. Maaza, A. Gurib-Fakim, Physical properties of CdO nanoparticles synthesized by green chemistry via Hibiscus Sabdariffa flower extract. J. Alloys Comp. 655, 314 (2016). https://doi.org/10.1016/j.jallcom.2015.09.063
M. Bououdina, A.A. Dakhel, M. El-Hilo, D.H. Anjum, M.B. Kanound, S. Goumri-Said, Revealing a room temperature ferromagnetism in cadmium oxide nanoparticles: an experimental and first-principles study. RSC Adv. 5, 33233 (2015). https://doi.org/10.1039/c5ră9b
A.A. Dakhel, Effect of thermal annealing in different gas atmospheres on the structural, optical and electrical properties of Li-doped CdO nanocrystalline films. Solid State Sci. 13, 1000 (2011). https://doi.org/10.1016/j.solidstatesciences.2011.02.002
R.K. Gupta, Z. Serbetci, F. Yakuphanoglu, Bandgap variation in size controlled nanostructured Li–Ni co-doped CdO thin films. J. Alloys Comp. 515, 96 (2012). https://doi.org/10.1016/j.jallcom.2011.11.098
B. Sahin, F. Bayansal, Facile synthesis of group-I elements (K, Li and Na)-doped nanostructured CdO films. Phil. Mag. 94, 4171 (2014). https://doi.org/10.1080/14786435.2014.981607
P. Velusamy, R. Ramesh Babu, K. Ramamurthi, Structural, morphological, optical and electrical properties of spray deposited lithium doped CdO thin films. Published by the American Institute of Physics DAE Solid State Physics Symposium 2015 AIP Conf. Proc. 1731, 080022 (2015). https://doi.org/10.1063/1.4947900
V.P. Zhukov, V.N. Krasil’nikov, A.P. Tyutyunnik, T.V. Dyachkova, N.A. Zhuravlev, A.V. Skachkov, T.A. Denisova, I.R. Shein, Impurity centers and electronic band structure of lithium-doped cadmium oxide. Ceram. Intern. 44, 17313 (2018). https://doi.org/10.1016/j.ceramint.2018.06.193
A. Das, C.P. Saini, D. Singh, R. Ahuja, A. Kaur, S. Aliukov, D. Shukla, F. Singh, High temperature mediated rocksalt to wurtzite phase transformation in cadmium oxide nano-sheets and their theoretical evidence. Nanoscale 11, 14802 (2019). https://doi.org/10.1039/c9nr01832h
M.H. Hassouni, K.A. Mishjil, S.S. Chiad, N.F. Habubi, Effect of gamma irradiation on the optical properties of Mg doped CdO thin films deposited by spray pyrolysis. Intern. Lett. Chem. Phys. Astron. 16, 26 (2013). https://doi.org/10.18052/www.scipress.com/ILCPA.16.26
A.A. Dakhel, Development of electrical conduction with beryllium doping of CdO nanostructure thin films. Mater. Res. 18, 222 (2015). https://doi.org/10.1590/1516-1439.301014
L. Ran, G. Lin-Jie, L. Long-Jiang, Z. Sheng-Jun, W. Jiang-Long, F. Guang-Sheng, W. Shu-Fang, High temperature thermoelectric performance of Ca2+doped CdO ceramics. Acta Phys. Sin. 64, 218101 (2015). https://doi.org/10.7498/aps.64.218101
L. Gao, J. Wang, L. Li, S. Wang, S. Zhai, S. Liang, G. Fu, Tuning of the microstructure and thermoelectric properties of CdO ceramics by Mg substituting. Mater. Chem. Phys. 174, 172 (2016). https://doi.org/10.1016/j.matchemphys.2016.02.069
L. Gao, S. Wang, R. Liu, X. Zha, N. Sun, S. Wang, J. Wang, G. Fu, Enhanced thermoelectric performance in Mg and Ca substituted CdO ceramics. RSC Adv. 6, 42249 (2016). https://doi.org/10.1039/c6ra04175b
A.A. Dakhel, Influence of dysprosium doping on the electrical and optical properties of CdO thin films. Sol. Energy 83, 934 (2009). https://doi.org/10.1016/j.solener.2008.12.015
A.A. Dakhel, Band gap narrowing in CdO doped with europium. Optical Mater. 31, 691 (2009). https://doi.org/10.1016/j.optmat.2008.08.001
S. Wang, F. Liu, Q. Lu, S. Dai, J. Wang, W. Yu, G. Fu, The effect of Er3+ doping on the structure and thermoelectric properties of CdO ceramics. J. Eur. Ceram. Soc. 33, 1763 (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.02.025
A. Alemi, S.W. Joo, S. Khademinia, M. Dolatyari, A. Bakhtiari, H. Moradi, S. Saeidi, Sol-gel synthesis, characterization, and optical properties of Gd3+-doped CdO sub-micron materials. Intern. Nano Lett. 3, 41 (2013). https://doi.org/10.1186/2228-5326-3-41
H.Y. He, J. Lu, Band gap narrowing of cadmium oxide powder by rare earth praseodymium doping. MRS Commun. 3, 47 (2013). https://doi.org/10.1557/mrc.2013.6
S. Wang, Q. Lu, L. Li, G. Fu, F. Liu, S. Dai, W. Yu, J. Wang, High-temperature thermoelectric properties of Cd1-xPrxO ceramics. Scripta Mater. 69, 533 (2013). https://doi.org/10.1016/j.scriptamat.2013.06.018
A. Alemi, S. Khademinia, S.W. Joo, M. Dolatyari, A. Bakhtiari, H. Moradi, A. Esmaeilzadeh, Sol-gel synthesis, structural and optical properties investigation of Ce4+ doped CdO sub-micron materials. J. Appl. Chem. 8, 35 (2014)
M. Ravikumar, V. Ganesh, M. Shkir, R. Chandramohan, K.D.A. Kumar, S. Valanarasu, A. Kathalingam, S. AlFaify, Fabrication of Eu doped CdO [Al/Eu-nCdO/p-Si/Al] photodiodes by perfume atomizer based spray technique for opto-electronic applications. J. Mol. Struct. 1160, 311 (2018). https://doi.org/10.1016/j.molstruc.2018.01.095
S. Jin, Y. Yang, J.E. Medvedeva, J.R. Ireland, A.W. Metz, J. Ni, C.R. Kannewurf, A.J. Freeman, T.J. Marks, Dopant ion size and electronic structure effects on transparent conducting oxides. Sc-doped CdO thin films grown by MOCVD. J. Am. Chem. Soc. 126, 13787 (2004). https://doi.org/10.1021/ja0467925.
K.R. Murali, A. Kalaivanan, S. Perumal, N.N. Pillai, Sol-gel dip coated CdO: Al films. J. Alloys Comp. 503, 350 (2010). https://doi.org/10.1016/j.jallcom.2009.11.187
S. Jin, Y. Yang, J.E. Medvedeva, L. Wang, S. Li, N. Cortes, J.R. Ireland, A.W. Metz, J. Ni, M.C. Hersam, A.J. Freeman, T.J. Marks, Tuning the properties of transparent oxide conductors. Dopant ion size and electronic structure effects on CdO-based transparent conducting oxides. Ga- and In-doped CdO thin films grown by MOCVD. Chem. Mater. 20, 220 (2008). https://doi.org/10.1021/cm702588m
R. Kumaravel, K. Ramamurthi, V. Krishnakumar, Effect of indium doping in CdO thin films prepared by spray pyrolysis technique. J. Phys. Chem. Solids. 71, 1545 (2010). https://doi.org/10.1016/j.jpcs.2010.07.021
A.A. Dakhel, Effect of thallium doping on the electrical and optical properties of CdO thin films. Phys. Stat. Sol. 205, 2704 (2008). https://doi.org/10.1002/pssa.200723472
S. Sahare, R.K. Choubey, G. Jadhav, T.M. Bhave, S. Mukherjee, S. Kumar, A Comparative investigation of optical and structural properties of Cu-doped CdO-derived nanostructures. J. Supercond. Nov. Magn. 30, 1439 (2017). https://doi.org/10.1007/s10948-016-3943-y
G. Fu, L. Gao, R. Liu, X. Zha, J. Wang, S. Wang, Synergistically tuning the electrical and thermal transport properties of CdO: Cu thermoelectric ceramics. Mater. Res. Express. 4, 075502 (2017). https://doi.org/10.1088/2053-1591/aa70d8
X.Y. Zha, L.J. Ga, H.C. Bai, J.L. Wang, S.F. Wang, Optimize the thermoelectric performance of CdO ceramics by doping Zn. Chin. Phys. B. 10, 107202 (2017). https://doi.org/10.1088/1674-1056/26/10/107202
A. Salem, Silver-doped cadmium oxide nanoparticles: Synthesis, structural and optical properties. Eur. Phys. J. Plus. 129, 263 (2014). https://doi.org/10.1140/epjp/i2014-14263-3
M.R. Alam, M.M. Rahman, A.M.M. Tanveer Karim, M.K.R. Khan, Effect of Ag incorporation on structural and opto electric properties of pyrolized CdO thin films. Intern. Nano Lett. 8, 287 (2018). https://doi.org/10.1007/s40089-018-0251-5
A.A. Dakhel, Electrical and optical properties of iron-doped CdO. Thin Solid Films 518, 1712 (2010). https://doi.org/10.1016/j.tsf.2009.11.026
N. Rajkumar, V.M. Susila, K. Ramachandran, On the possibility of ferromagnetism in CdO: Mn at room temperature. J. Exp. Nanosci. 6, 389 (2011). https://doi.org/10.1080/17458080.2010.497969
F. Yakuphanoglu, Preparation of nanostructure Ni doped CdO thin films by sol gel spin coating method. J. Sol-Gel Sci. Technol. 59, 569 (2011). https://doi.org/10.1007/s10971-011-2528-2
L.V.K. Rao, D.V. Sathish, C.V. Reddy, U.S.U. Thampy, K. Venkateswarlu, P.S. Rao, R.V.S.S.N. Ravikumar, Structural properties of Cr3+-doped cadmium oxide nanopowders. Appl. Magn. Reson. 42, 403 (2012). https://doi.org/10.1007/s00723-011-0308-3
H. Colak, O. Turkoglu, Synthesis and characterization of Fe-Doped CdO binary system, J. Ceram. Process. Res. 14, 616 (2013). https://doi.org/10.36410/jcpr.2013.14.5.616
T. Ahmad, S. Khatoon, K. Coolahan, S.E. Lofland, Solvothermal synthesis, optical and magnetic properties of nanocrystalline Cd1-xMnxO (0.04<x=0.10) solid solutions. J. Alloys Compd. 558, 117 (2013). https://doi.org/10.1016/j.jallcom.2012.12.159
T. Ahmad, S. Khatoon, Structural characterization, optical and magnetic properties of Ni-doped CdO dilute magnetic semiconductor nanoparticles. J. Mater. Res. 28, 1245 (2013). https://doi.org/10.1557/jmr.2013.69
Z.Q. Liu, R. Guo, G.R. Li, Q. Bu, W.X. Zhao, Y.X. Tong, Facile electrodeposition of large-area CdO and Cd1-xCoxO curved nanowires. Electrochim. Acta. 59, 449 (2012). https://doi.org/10.1016/j.electacta.2011.10.095
T. Ahmad, S. Khatoon, S.E. Lofland, G. Thakur, Structural characterization and properties of nano-sized Cd1-xCoxO dilute magnetic semiconductors prepared by solvothermal method. Mater. Sci. Semicond. Proc. 17, 207 (2014). https://doi.org/10.1016/j.mssp.2013.09.025
S. Kumar, S. Layek, M. Yashpal, A.K. Ojha, Room temperature ferromagnetism in undoped and Mn doped CdO nanostructures. J. Magn. Magn. Mater. 393, 555 (2015). https://doi.org/10.1016/j.jmmm.2015.06.011
R. Ranjithkumar, A.A. Irudayaraj, G. Jayakumar, A.D. Raj, S. Karthick, R. Vinayagamoorthy, Synthesis and Properties of CdO and Fe doped CdO Nanoparticles. Materials Today: Proceedings. 3, 1378 (2016). https://doi.org/10.1016/j.matpr.2016.04.018
W.Z. Tawfik, M. Esmat, S.I. El-Dek, Drastic improvement in magnetization of CdO nanoparticles by Fe doping. Appl. Nanosci. 7, 863 (2017). https://doi.org/10.1007/s13204-017-0591-x
D.J. Jeejamol, A.M.E. Raj, K. Jayakumari, C. Ravidhas, Optimization of CdO nanoparticles by Zr4+ doping for better photocatalytic activity. J. Mater. Sci.: Mater. Electron. 29, 97 (2018). https://doi.org/10.1007/s10854-017-7893-3
L.A. Wahab, H.A. Zayed, I.S. Yahia, N.M. Yousif, Structural and optical properties of some metals doped CdO synthesized by sol-gel. J. Scientific Res. Sci. 32, 391 (2015). https://doi.org/10.21608/jsrs.2015.18837
N.S. Gajbhiye, R.S. Ningthoujam, A. Ahmed, D.K. Panda, S.S. Umre, S.J. Sharma, Re-dispersible Li+ and Eu3+ co-doped CdO nanowires: Luminescence studies. Proc. of ASID ’06, New Delhi 8–12 Oct, 2006, pp. 275–277
A.A. Dakhel, M. El-Hilo, M. Bououdina, Cu-Codoping for the enhancement of ferromagnetism of Fe-doped CdO nanopowders. J. Supercond. Nov. Magn. 27, 2089 (2014). https://doi.org/10.1007/s10948-014-2553-9
A.A. Dakhel, M. Bououdina, Development of transparent conducting copper and iron co-doped cadmium oxide films: Effect of annealing in hydrogen atmosphere. Mater. Sci. Semicond. Proc. 26, 527 (2014). https://doi.org/10.1016/j.mssp.2014.05.015
M. Bououdina, A.A. Dakhel, Creation of RT-FM in CdO nanocrystalline powder by codoping with Cu and Gd: Effect of annealing in hydrogen atmosphere. J. Alloys Compd. 601, 162 (2014). https://doi.org/10.1016/j.jallcom.2014.02.146
R. Aydin, B. Sahin, Comprehensive research on physical properties of Zn and M (M: Li, Na, K) double doped cadmium oxide (CdO) nanostructures using SILAR method. Ceram. Intern. 43, 9285 (2017). https://doi.org/10.1016/j.ceramint.2017.04.087
R. Aydin, H. Cavusoglu, B. Sahin, Transition metal Mn/Cu co-doped CdO transparent conductive films: Effect on structural, morphological and optical characteristics. J. Alloys Compd. 785, 523 (2019). https://doi.org/10.1016/j.jallcom.2019.01.221
R. Aydin, B. Sahin, Li: Ce co-doped CdO films synthesized by SILAR method: Effects of rare earth element Ce content on the physical attributes. Ceram. Intern. 44, 22249 (2018). https://doi.org/10.1016/j.ceramint.2018.08.346
H. Cavusoglu, R. Aydin, B. Sahin, A comparative study on cobalt and aluminum as a dual doping element for CdO films. Ceram. Intern. 45, 899 (2019). https://doi.org/10.1016/j.ceramint.2018.09.265
R.H. Meinhold, Studies of impure cadmium oxide semiconductors using NMR, EPR and conductivity measurements. J. Phys. Chem. Solids. 48, 927 (1987). https://doi.org/10.1016/0022-3697(87)90129-6
K.R. Kishore, D. Balamurugan, B.G. Jeyaprakash, Electrospinning based CdO nanograins for formaldehyde vapour detection by chemiresistive method. Mater. Sci. Semicond. Proc. 121, 105296 (2021). https://doi.org/10.1016/j.mssp.2020.105296
V.N. Krasil’nikov, I.V. Baklanova, V.P. Zhukov, O.I. Gyrdasova, T.V. Dyachkova, A.P. Tyutyunnik, Thermally stimulated infrared shift of cadmium oxide optical absorption band edge. Mater. Sci. Semicond. Proc. 124, 105605 (2021). https://doi.org/10.1016/j.mssp.2020.105605
A.A. Dakhel, Structural, optical, and magnetic studies of iodine-doped CdO nanopowders and films. J. Sol-Gel Sci. Technol. 85, 311 (2018). https://doi.org/10.1007/s10971-017-4567-9
V.P. Zhukov, N.I. Medvedeva, V.N. Krasil’nikov, First-principles study of intrinsic defects in CdO. Intern. J. Modern Phys. B. 32, 1850059 (2018). https://doi.org/10.1142/S0217979218500595
V.N. Krasil’nikov, T.V. Dyachkova, A.P. Tyutyunnik, O.I. Gyrdasova, Yu.A. Perevozchikova, V.V. Marchenkov, H.W. Weber, Precursor synthesis and magnetic properties of Cd1-xFexO (0≤x≤0.07). Mendeleev Commun. 27, 456 (2017). https://doi.org/10.1016/j.mencom.2017.09.008
V.N. Krasil’nikov, V.P. Zhukov, O.I. Gyrdasova, T.V. Dyachkova, A.P. Tyutyunnik, Yu.A. Perevozchikova, H.W. Weber, A. Zaleski, V.V. Marchenkov, Precursor synthesis and magnetic properties of Cd1-xFexO (≤x≤0.07) polycrystalline solid solutions. J. Alloys Comp. 725, 1244 (2017). https://doi.org/10.1016/j.jallcom.2017.07.255
R. Chaurasiya, A. Dixit, Point defects induced magnetism in CdO monolayer: A theoretical study. J. Magn. Magn. Mater. 469, 279 (2019). https://doi.org/10.1016/j.jmmm.2018.08.076
I.G. Morozov, O.V. Belousova, M.V. Kuznetcov, Room temperature ferromagnetism in nanostructured submicron Cd/CdO particles. J. Mater. Sci.: Mater. Electron. 31, 6664 (2020). https://doi.org/10.1007/s10854-020-03222-z
N.N. Jandow, M.S. Othman, N.F. Habubi, S.S. Chiad, K.A. Mishjil, I.A. Al-Baidhany, Theoretical and experimental investigation of structural and optical properties of lithium doped cadmium oxide thin films. Mater. Res. Express. 6, 116434 (2019). https://doi.org/10.1088/2053-1591/ab4af8
K. Sankarasubramanian, P. Soundarrajan, K. Sethuraman, K. Ramamurthi, Chemical spray pyrolysis deposition of transparent and conducting Fe doped CdO thin films for ethanol sensor. Mater. Sci. Semicond. Proc. 40, 879 (2015). https://doi.org/10.1016/j.mssp.2015.07.090
C. Aydın, O.A. Al-Hartomy, A.A. Al-Ghamdi, F. Al-Hazmi, I.S. Yahia, F. El-Tantawy, Controlling of crystal size and optical band gap of CdO nanopowder semiconductors by low and high Fe contents. J. Electroceram. 29, 155 (2012). https://doi.org/10.1007/s10832-012-9748-x
A. Wang, J.R. Babcock, N.L. Edleman, A.W. Metz, M.A. Lane, R. Asahi, V.P. Dravid, C.R. Kannewurf, A.J. Freeman, T.J. Marks, Indium-cadmium-oxide films having exceptional electrical conductivity and optical transparency: Clues for optimizing transparent conductors. Proc. Natl. Acad. Sci. 98, 7113 (2001). https://doi.org/10.1073/pnas.121188298
J.A. Bastin, R.W. Wright, The electrical conductivity of cadmium oxide at low temperatures. Proc. Phys. Soc. A. 68, 312 (1955). https://doi.org/10.1016/j.mssp.2015.07.090
R.D. Pierce,S.A. Friedberg, Heat Capacities of Fe(HCOO)2 2H2O and Ni(HCOO)2 2H2O between 1.4 and 20 K. Phys. Rev. B. 3, 934 (1971). https://doi.org/10.1103/PhysRevB.3.934
B. Malecka, A. Lacz, Thermal decomposition of cadmium formate in inert and oxidative atmosphere. Thermochim. Acta. 479, 12 (2008). https://doi.org/10.1016/j.tca.2008.09.003
K. Mouaine, P. Becker, C. Carabatos-Nedelec, Thermal and spectroscopic study of dehydration of lithium formate monohydrate single-crystals. J. Therm. Anal. Calorim. 55, 807 (1999). https://doi.org/10.1023/A:1010125631748
K. Muraishi, T. Takano, K. Nagase, N. Tanaka, Thermal decomposition of Fe(II) carboxylates: comparison of decomposition processes between the formate and malonate. J. Inorg. Nucl. Chem. 43, 2293 (1981). https://doi.org/10.1016/0022-1902(81)80252-7
G. Williamson, W. Hall, X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1, 22 (1953). https://doi.org/10.1016/0001-6160(53)90006-6
W. Kraus, G. Nolze, POWDER CELL - a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 29, 301 (1996). https://doi.org/10.1107/S0021889895014920
A.N. Cherepanov, V.V. Marchenkov, V.E. Startsev, N.V. Volkenshtein, M. Glin'ski, High-field galvanomagnetic properties of compensated metals under electron-surface and intersheet electron-phonon scattering (tungsten). J. Low Temp. Phys. 80, 135 (1990). https://doi.org/10.1007/BF00683481
C. Jacoboni, Theory of electron transport in semiconductors, (Springer Series in Solid-State Sciences, 2010), V. 165, p. 588. https://doi.org/10.1007/978-3-642-10586-9
A.A. Ziabari, F.E. Ghodsi, G. Kiriakidis, Correlation between morphology and electro-optical properties of nanostructured CdO thin films: Influence of Al doping. Surf. Coat. Technol. 213, 15 (2012). https://doi.org/10.1016/j.surfcoat.2012.10.003
S.D. Sarma, E.H. Hwang, A. Kaminski, Temperature-dependent magnetization in diluted magnetic semiconductors. Phys. Rev. B. 67, 155201 (2003). https://doi.org/10.1103/PhysRevB.67.155201
V.N. Krasilnikov, V.P. Zhukov, O.I. Gyrdasova, A.P. Tyutyunnik, T.V. Dyachkova, Yu.A. Perevozchikova, F. Sauerzopf, V.V. Marchenkov, Precursor synthesis, magnetic properties and electronic band structure of Mg1-xFexO (0<x<0.075). J. Alloys Compd. 789, 30–39 (2019). https://doi.org/10.1016/j.jallcom.2019.03.021
Acknowledgements
The research was carried out within the state assignment of the Ministry of Education and Science of the Russian Federation (theme “Spin”, No. AAAA-A18-118020290104-2), supported in part by “Electrical Engineering” Shanghai class 2 Plateau Discipline, the Government of the Russian Federation (Decree No. 211, Contract No. 02.A03.21.0006), “Electrical Engineering” Shanghai class 2 Plateau Discipline and the National Natural Science Foundation of China (NSFC, Nos. 12074242, 51862032). Absorption spectra were obtained using the equipment at the Center for Joint Use "Spectroscopy and Analysis of Organic Compounds" at the Postovsky Institute of Organic Synthesis, UB RAS. The optical measurements were carried out in accordance with the scientific and research plans and state assignment of the Institute of Solid State Chemistry, UB RAS (Grant No. AAAA-A19-119031890025-9). E.V.C. acknowledges funding by Saint Petersburg State University project for scientific investigations (ID No. 73028629). The calculations were performed on the URAN cluster at the Institute of Mathematics and Mechanics of the Urals Branch in RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krasil’nikov, V., Zhukov, V., Chulkov, E. et al. Precursor synthesis and properties of iron and lithium co-doped cadmium oxide. J Electroceram 48, 127–142 (2022). https://doi.org/10.1007/s10832-022-00278-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-022-00278-7