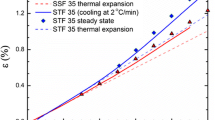
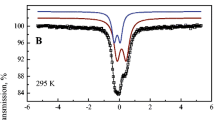
Abstract
The bulk electrical conductivity of the mixed ionic-electronic conducting perovskite-structured SrSn1-xFexO3-x/2+δ (SSF) was measured to examine how changes in defect chemistry and electronic band structure associated with the substitution of Ti by Sn impact defect charge carrier density and ultimately electrode performance. These results complement a defect chemical model for SSF investigated and reported in Part I of this study. The electrical properties of SSF were found not to differ significantly from the corresponding composition in SrTi1-xFexO3-x/2+δ (STF). It is believed that Fe dominates the character of the valence and conduction bands and thus governs the electronic properties in SSF. Though slightly shifted in energy due to the larger size of Sn, the defect equilibria and therefore the electrical conductivity of SSF were found to be largely dominated by Fe and thus differed only in a limited way from that in STF. Key kinetic parameters obtained include the migration enthalpy of oxygen vacancies (0.772 ± 0.204 eV), the activation energy of area-specific-resistance for oxygen exchange (1.65 ± 0.03 eV) and the magnitudes of electron (0.0002 ± 0.00005 cm2/V∙s) and hole (0.0037 ± 0.0015 cm2/V∙s) mobilities.











Similar content being viewed by others
References
W. Jung, H.L. Tuller, A new model describing solid oxide fuel cell cathode kinetics: Model thin film SrTi1-xFexO3-δ mixed conducting oxides–a case study. Adv. Energy Mater. 1, 1184–1191 (2011)
A. Rothschild, W. Menesklou, H.L. Tuller, E. Ivers-Tiffée, Electronic structure, defect chemistry, and transport properties of SrTi1-xFexO3-y solid solutions. Chem. Mater. 18(16), 3651–3659 (2006)
C.S. Kim, S.R. Bishop, N.H. Perry, H.L. Tuller, Electro-chemo-mechanical studies of perovskite-structured mixed ionic-electronic conducting SrSn1-xFexO3-x/2+δ part I: Defect chemistry. J. Electroceram. 38(1), 74–80 (2017)
H.J. Kim, U. Kim, T.H. Kim, J. Kim, H.M. Kim, B.G. Jeon, W.J. Lee, H.S. Mun, K.T. Hong, J. Yu, K. Char, K.H. Kim, Physical properties of transparent perovskite oxides (Ba,La)SnO 3 with high electrical mobility at room temperature. Phys. Rev. B - Condens. Matter Mater. Phys. 86(16), 1–9 (2012)
D.J. Singh, D.A. Papaconstantopoulos, J.P. Julien, F. Cyrot-Lackmann, Electronic structure of Ba(Sn,Sb) O3: Absence of superconductivity. Phys. Rev. B 44(17), 9519–9523 (1991)
V. Thangadurai, P. Schmid Beurmann, W. Weppner, Mixed oxide ion and electronic conductivity in perovskite-type SrSnO3 by Fe substitution. Mater. Sci. Eng. B 100(1), 18–22 (2003)
V. Thangadurai, R.A. Huggins, W. Weppner, Use of simple ac technique to determine the ionic and electronic conductivities in pure and Fe-substituted SrSnO3 perovskites. J. Power Sources 108(1–2), 64–69 (2002)
M. Kuhn, J.J. Kim, S.R. Bishop, H.L. Tuller, Oxygen Nonstoichiometry and defect chemistry of perovskite-structured BaxSr1– xTi1–yFeyO3–y/2+δ solid solutions. Chem. Mater. 25(15), 2970–2975 (2013)
K.S. Roh, K.H. Ryu, C.H. Yo, Nonstoichiometry and physical properties of the SrSn1−xFexO3−ySystem. J. Solid State Chem. 142(2), 288–293 (1999)
A. Rothschild, S. J. Litzelman, H. L. Tuller, W. Menesklou, T. Schneider, and E. Ivers-Tiffée, Temperature-independent resistive oxygen sensors based on SrTi1-xFexO3-δ solid solutions. Sensors Actuators, B Chem., vol. 108, no. 1–2 SPEC. ISS., pp. 223–230, 2005.
D. Chen, S.R. Bishop, H.L. Tuller, Praseodymium-cerium oxide thin film cathodes: Study of oxygen reduction reaction kinetics. J. Electroceram. 28(1), 62–69 (2012)
H.L. Tuller, A.S. Nowick, Small polaron electron transport in reduced CeO2 single crystals. J. Phys. Chem. Solids 38(8), 859–867 (1977)
C.G. Fonstad, R.H. Rediker, Electrical properties of high-quality stannic oxide crystals. J. Appl. Phys. 42(7), 2911–2918 (1971)
Q. Liu, H. Li, B. Li, W. Wang, Q. Liu, Y. Zhang, and J. Dai, “Structure and band gap engineering of Fe-doped SrSnO 3 epitaxial films,” EPL (Europhysics Lett., vol. 108, no. 3, p. 37003, 2014.
V. Metlenko, W. Jung, S.R. Bishop, H.L. Tuller, R.A. De Souza, Oxygen diffusion and surface exchange in the mixed conducting oxides SrTi1-yFeyO3-δ. Phys. Chem. Chem. Phys. 18(42), 29495–29505 (2016)
M. Cherry, M.S. Islam, C.R.A. Catlow, Oxygen ion migration in perovskite-type oxides. J. Solid State Chem. 118(1), 125–132 (1995)
F. Baumann, J. Fleig, H. Habermeier, J. Maier, Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ionics 177(11–12), 1071–1081 (2006)
W. Jung, H.L. Tuller, Investigation of surface Sr segregation in model thin film solid oxide fuel cell perovskite electrodes. Energy Environ. Sci. 5(1), 5370 (2012)
C. Körber, A. Wachau, P. Agoston, K. Albe, A. Klein, Self-limited oxygen exchange kinetics at SnO2 surfaces. Phys. Chem. Chem. Phys. 13(8), 3223–3226 (2011)
W. Lee, J.W. Han, Y. Chen, Z. Cai, B. Yildiz, Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 135(21), 7909–7925 (2013)
K.-C. Chang, P. Fuoss, Y. Hoydoo, S. Gopalan, D. Ding, L. Meilin, B. Yildiz, and K. Gerdes, Recent Solid Oxide Fuel Cell Cathode Studies, p. 159, 2013.
N. Caillol, M. Pijolat, E. Siebert, Investigation of chemisorbed oxygen, surface segregation and effect of post-treatments on La0.8Sr0.2MnO3 powder and screen-printed layers for solid oxide fuel cell cathodes. Appl. Surf. Sci. 253(10), 4641–4648 (2007)
Acknowledgments
This research was carried out as a part of the activity of the Skoltech-MIT Center for Electrochemical Energy Storage. Some of the concepts applied in this study on SSF were developed previously in research supported by the National Science Foundation under award number DMR-1507047.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, C.S., Bishop, S.R. & Tuller, H.L. Electro-chemo-mechanical studies of perovskite-structured mixed ionic-electronic conducting SrSn1-xFexO3-x/2+δ part II: Electrical conductivity and cathode performance. J Electroceram 40, 57–64 (2018). https://doi.org/10.1007/s10832-017-0098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-017-0098-6