Abstract
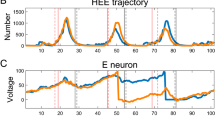
Neuronal systems are subject to rapid fluctuations both intrinsically and externally. These fluctuations can be disruptive or constructive. We investigate the dynamic mechanisms underlying the interactions between rapidly fluctuating signals and the intrinsic properties of the target cells to produce variable and/or coherent responses. We use linearized and non-linear conductance-based models and piecewise constant (PWC) inputs with short duration pieces. The amplitude distributions of the constant pieces consist of arbitrary permutations of a baseline PWC function. In each trial within a given protocol we use one of these permutations and each protocol consists of a subset of all possible permutations, which is the only source of uncertainty in the protocol. We show that sustained oscillatory behavior can be generated in response to various forms of PWC inputs independently of whether the stable equilibria of the corresponding unperturbed systems are foci or nodes. The oscillatory voltage responses are amplified by the model nonlinearities and attenuated for conductance-based PWC inputs as compared to current-based PWC inputs, consistent with previous theoretical and experimental work. In addition, the voltage responses to PWC inputs exhibited variability across trials, which is reminiscent of the variability generated by stochastic noise (e.g., Gaussian white noise). Our analysis demonstrates that both oscillations and variability are the result of the interaction between the PWC input and the target cell’s autonomous transient dynamics with little to no contribution from the dynamics in vicinities of the steady-state, and do not require input stochasticity.










Similar content being viewed by others
References
Allen, E. J., Novosel, S. J., & Zhang, Z. (1998). Finite element and difference approximation of some linear stochastic partial differential equations. Stochastics and Stochastic Reports, 64, 117–142.
Amit, D. J. (1989). Modeling brain function: The world of attractor neural networks. New York, NY: Cambridge University Press.
Anishchenko, V. S., & Neiman, B. (1997). Stochastic synchronization. Stochastic Dynamics (in Lecture Notes Physics), 484, 154–166.
Arieli, A., Sterkin, A., Grinvald, A., & Aertsen, A. D. (1996). Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science, 273(5283), 1868–1871.
Baltanas, J. P., & Casado, J. M. (1998). Bursting behavior of the Fitzhugh-Nagumo neuron model subject to quasi-monochromatic noise. Physica D: Nonlinear Phenomena, 122, 231–240.
Baspinar, E., Schulen, L., Olmi, S., & Zakharova, A. (2021). Coherence resonance in neuronal populations: Mean-field versus network model. Physical Review E, 103, 032308.
Benzi, R., Parisi, G., Sutera, A., & Vulpiani, A. (1982). Stochastic resonance in climatic change. Tellus, 34, 10–16.
Bernstein, J. G., & Boyden, E. S. (2012). Optogenetic tools for analyzing the neural circuits of behavior. Current Opinion in Neurobiology, 22, 61–71.
Bondanelli, G., & Ostojic, S. (2020). Coding with transient trajectories in recurrent neural networks. PLoS Computational Biology, 16, e1007655.
Boucheny, C., Brunel, N., & Arleo, A. (2005). A continuous attractor network model without recurrent excitation: maintenance and integration in the head direction cell system. Journal of Computational Neuroscience, 18, 205–227.
Britten, K. H., Shadlen, M. N., Newsome, W. T., & Movshon, J. A. (1992). The analysis of visual motion: a comparison of neuronal and psychophysical performance. Journal of Neuroscience, 12(12), 4745–4765.
Burden, R. L., & Faires, J. D. (1980). Numerical analysis. PWS Publishing Company - Boston.
Brunel, N., Chance, F. S., Fourcaud, N., & Abbott, L. F. (2001). Effects of synaptic noise and filtering on the frequency response of spiking neurons. Physical Review Letters, 86, 2186–2189.
Calvin, W. H., & Stevens, C. F. (1967). Synaptic noise as a source of variability in the interval between action potentials. Science, 155, 842–844.
Chow, C. C., & White, J. A. (1996). Spontaneous action potentials due to channel fluctuation. Biophysical Journal, 71, 3013–3021.
Churchland, M. M., Byron, M. Y., Ryu, S. I., Santhanam, G., & Shenoy, K. V. (2006). Neural variability in premotor cortex provides a signature of motor preparation. Journal of Neuroscience, 26(14), 3697–3712.
Churchland, M. M., Yu, B. M., Cunningham, J. P., Sugrue, L. P., Cohen, M. R., Corrado, G. S., et al. (2010). Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nature Neuroscience, 13, 369–378.
Cohen, M. R., & Kohn, A. (2011). Measuring and interpreting neuronal correlations. Nature Neuroscience, 14(7), 811–819.
Collins, J. J., Chow, C. C., & Imhoff, T. T. (1995). Aperiodic stochastic resonance in excitable systems. Physical Review E, 76, 642–645.
Collins, J. J., Imhoff, T. T., & Grigg, P. (1996). Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. Journal of Neurophysiology, 76, 642–645.
Day, J., Rubin, J. E., & Chow, C. C. (2009). Competition between transients in the rate of approach to a fixed point. SIAM Journal on Applied Dynamical Systems, 8(4), 1523–1563.
Deco, G., Rolls, E., & Romo, R. (2009). Stochastic dynamics as a principle of brain function. Progress in Neurobiology, 88, 1–16.
DeFelice, L. J. (1981). Introduction to channel noise. Plenum Press.
Deisseroth, K. (2011). Optogenetics. Nature Methods, 8, 26–29.
Destexhe, A., Badoual, M., Piwkowska, Z., Bal, T., & Rudolph, M. (2004). A novel method for characterizing synaptic noise in cortical neurons. Neurocomputing, 58, 191–196.
Destexhe, A., & Rudolph-Lilith, M. (2012). Neuronal Noise. Springer.
DeVille, R. L., Vanden-Eijnden, E., & Muratov, C. B. (2005). Two distinct mechanisms of coherence in randomly perturbed dynamical systems. Physical Review E, 72, 031105.
Dorval, A. D., Jr., & White, J. A. (2005). Channel noise is essential for perithreshold oscillations in entorhinal stellate neurons. Journal of Neuroscience, 25, 10025–10028.
Douglass, J. K., Wilkens, L., Pantazelou, E., & Moss, F. (1993). Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature, 365, 337–340.
Du, Q., & Zhang, T. (2002). Numerical approximation of some linear stochastic partial differential equations driven by special additive noise. SIAM Journal on Numerical Analysis, 400, 1421–1445.
Faisal, A. A., Selen, L. P., & Wolpert, D. M. (2008). Noise in the nervous system. Nature Reviews Neuroscience, 9(4), 292–303.
Fellous, J. M., Rudolph, M., Destexhe, A., & Sejnowski, T. J. (2003). Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience, 122, 811–829.
Fernandez, R., & White, J. A. (2008). Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. Journal of Neuroscience, 28, 3790–3803.
Fox, M. D., Snyder, A. Z., Zacks, J. M., & Raichle, M. E. (2006). Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature Neuroscience, 9(1), 23–25.
Gammaitoni, L., Hanggi, P., Jung, P., & Marchesoni, F. (1998). Stochastic resonance. Reviews of Modern Physics, 70, 223–287.
Gardiner, C. W. (1985). Handbook of Stochastic Methods. Berlin: Springer-Verlag.
Guckenheimer, J., & Holmes, P. (1983). Nonlinear oscillations, dynamical systems, and bifurcations of vector fields. New York: Springer-Verlag.
Hakim, V., & Rappel, W.-J. (1994). Noise-induced periodic behaviour in the globally coupled complex Ginzburg-Landau equation. Europhysics Letters, 27, 637–642.
Hong, S., Ratté, S., Prescott, S. A., & De Schutter, E. (2012). Single neuron firing properties impact correlation-based population coding. Journal of Neuroscience, 32, 1413–1428.
Hopfield, J. J. (1982). Neural networks and physical systems with emergent collective computational abilities. Proceedings of the National academy of Sciences of the United States of America, 79, 2554–2558.
Hutcheon, B., Miura, R. M., & Puil, E. (1996). Subthreshold membrane resonance in neocortical neurons. Journal of Neurophysiology, 76, 683–697.
Hutcheon, B., & Yarom, Y. (2000). Resonance, oscillations and the intrinsic frequency preferences in neurons. Trends in Neurosciences, 23, 216–222.
Ito, T., Brincat, S. L., Mil, R. D., Siegel, M., He, B. J., Miller, E. K., et al. (2020). Task-evoked activity quenches neural correlations and variability in large-scale brain systems. PLoS Computational Biology, 16, e1007983.
Izhikevich, E. (2006). Dynamical Systems in Neuroscience: The geometry of excitability and bursting. MIT Press (Cambridge, Massachusetts).
Jensen, R. V. (1998). Synchronization of randomly driven nonlinear oscillators. Physical Review E, 58, R6907–R6910.
Knierim, J. J., & Zhang, K. (2012). Attractor dynamics of spatially correlated neural activity in the limbic system. Annual Review of Neuroscience, 32, 267–285.
Kurrer, C., & Schulten, K. (1995). Noise-induced synchronous neuronal oscillations. Physical Review E, 51, 6213–6218.
Laing, C., & Lord, G. J. (2010). Stochastic methods in neuroscience. Oxford University Press.
Lee, J., & Lee, J. (2018). Quantitative analysis of a transient dynamics of a gene regulatory network. Physical Review E, 98, 062404.
Lee, S.-G., Neiman, A., & Kim, S. (1998). Coherence resonance in a hodgkin-huxley neuron. Physical Review E, 57, 3292–3297.
Levenstein, D., Buzsaki, G., & Rinzel, J. (2019). Nrem sleep in the rodent neocortex and hippocampus reflects excitable dynamics. Nature Communications, 10, 2478.
Lim, S., & Rinzel, J. (2010). Noise-induced transitions in slow wave neuronal dynamics. Journal of Computational Neuroscience, 28, 1–17.
Lindner, B., García-Ojalvo, J., Neiman, A., & Schimansky-Geier, L. (2004). Effects of noise in excitable systems. Physics Reports, 392, 321–424.
Longtin, A., Bulsara, A., & Moss, F. (1991). Time-interval sequences in bistable systems and the noise-induced transmission of information by sensory neurons. Physical Review Letters, 67, 656.
Marin, B., Pinto, R. D., Elson, R. C., & Colli, E. (2014). Noise, transient dynamics, and the generation of realistic interspike interval variation in square-wave burster neurons. Physical Review E, 90, 042718.
Mato, G. (1989). Stochastic resonance using noise generated by a neural network. Physical Review E, 59, 3339–3343.
Matsumoto, K., & Tsuda, I. (1983). Noise-induced order. Journal of Statistical Physics, 31, 87–106.
Mazor, O., & Laurent, G. (2005). Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron, 48, 661–673.
McDonnell, M. D., & Abboott, D. (2009). What is stochastic resonance? definitions, misconceptions, debates, and its relevance to biology. PLoS Computational Biology, 5, e1000348.
McNamara, B., & Wiesenfeld, K. (1989). Theory of stochastic resonance. Physical Review A, 39, 4854–4869.
Middleton, J., Chacron, M., Lindner, B., & Longtin, A. (2003). Firing statistics of a neuron model driven by long-range correlated noise. Physical Review E, 68, 021920.
Muratov, C. B., Vanden-Eijnden, E., & Weinan, E. (2005). Self-induced stochastic resonance in excitable systems. Physica D: Nonlinear Phenomena, 210, 227–240.
Nachstedt, T., & Tetzlaff, C. (2016). Working memory requires a combination of transient and attractor-dominated dynamics to process unreliably timed inputs. Science and Reports, 7, 2473.
Neiman, A., Saparin, P. I., & Stone, L. (1997). Coherence resonance at noisy precursors of bifurcations in nonlinear dynamical systems. Physical Review E, 56, 270–273.
Pena, R. F. O., & Rotstein, H. G. (2022). The voltage and spiking responses of subthreshold resonant neurons to structured and fluctuating inputs: persistence and loss of resonance and variability. Biological Cybernetics, 116, 163-190.
Pena, R. F. O., Zaks, M. A., & Roque, A. C. (2018). Dynamics of spontaneous activity in random networks with multiple neuron subtypes and synaptic noise. Journal of Computational Neuroscience, 45, 1–28.
Pham, J., Pakdaman, K., & Vibert, J.-F. (1998). Noise-induced coherent oscillations in randomly connected neural networks. Physical Review E, 58, 3610–3622.
Pikovsky, A. S. (1984). Synchronization and stochastization of nonlinear oscillations by external noise. In: Nonlinear and Turbulent Processes in Physics, ed. Sagdeev, R. Z. Harwood Acad. Publ., 3:1601–1604.
Pikovsky, A. S., & Kurths, J. (1997). Coherence resonance in a noise-driven excitable system. Physical Review Letters, 78, 775–778.
Pradines, J. R., Osipov, G. V., & Collins, J. J. (1999). Coherence resonance in excitable and oscillatory systems: The essential role of slow and fast dynamics. Physical Review E, 60, 6407–6410.
Rabinovich, M., Huerta, R., & Laurent, G. (2008). Transient dynamics for neural processing. Science, 321, 48–50.
Rabinovich, M. I., & Varona, P. (2011). Robust transient dynamics and brain functions. Frontiers in Computational Neuroscience, 5, 24.
Rappel, W. J., & Karma, A. (1996). Noise-induced coherence in neural networks. Physical Review Letters, 77, 3251–3259.
Redish, A. D., Elga, A. N., & Touretzky, D. S. (1996). A coupled attractor model of the rodent head direction system. Network: Computation in Neural Systems, 7, 671–685.
Renart, A., & Machens, C. K. (2014). Variability in neural activity and behavior. Current Opinion in Neurobiology, 25, 211–220.
Renart, A., & Machens, C. K. (2014). Variability in neural activity and behavior. Current Opinion in Neurobiology, 25, 211–220.
Richardson, M. J. E. (2008). Spike-train spectra and network response functions for non-linear integrate-and-fire neurons. Biological Cybernetics, 99, 381–392.
Richardson, M. J. E., Brunel, N., & Hakim, V. (2003). From subthreshold to firing-rate resonance. Journal of Neurophysiology, 89, 2538–2554.
Risken, H. (1989). The Fokker-Planck equation (2nd ed.). Berlin: Springer-Verlag.
Robbe, L. T., Goris, J., Movshon, A., & EP, Smincelli. (2014). Partitioning neuronal variability. Nature Neuroscience, 17(6), 858–865.
Robinson, P. C., & Harsch, P. C. (2002). Stages of spike time variability during neuronal responses to transient inputs. Physical Review E, 66, 061902.
Romo, Ranulfo, Hernández, Adrián, Zainos, Antonio, & Salinas, Emilio. (2003). Correlated neuronal discharges that increase coding efficiency during perceptual discrimination. Neuron, 38(4), 649–657.
Rotstein, H. G. (2014). Frequency preference response to oscillatory inputs in two-dimensional neural models: a geometric approach to subthreshold amplitude and phase resonance. The Journal of Mathematical Neuroscience, 4, 11.
Rotstein, H. G. (2015). Subthreshold amplitude and phase resonance in models of quadratic type: nonlinear effects generated by the interplay of resonant and amplifying currents. Journal of Computational Neuroscience, 38, 325–354.
Rotstein, H. G., & Nadim, F. (2014). Frequency preference in two-dimensional neural models: a linear analysis of the interaction between resonant and amplifying currents. Journal of Computational Neuroscience, 37, 9–28.
Rotstein, H. G., Oppermann, T., White, J. A., & Kopell, N. (2006). The dynamic structure underlying subthreshold oscillatory activity and the onset of spikes in a model of medial entorhinal cortex stellate cells. Journal of Computational Neuroscience, 21, 271–292.
Rowat, P. F., & Elson, R. C. (2004). State-dependent effects of Na channel noise on neuronal burst generation. Journal of Computational Neuroscience, 16, 87–112.
Samsonovich, A., & McNaughton, B. L. (1997). Path integration and cognitive mapping in a continuous attractor neural network model. Journal of Neuroscience, 17, 5900–5920.
Schneidman, E., Freedman, B., & Segev, I. (1998). Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Computation, 10, 1679–1703.
Shadlen, M. N., & Newsome, W. T. (1998). The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. Journal of Neuroscience, 18, 3870–3896.
Shalinsky, J. H., Magistretti, J., Ma, L., & Alonso, A. A. (2002). Muscarinic activation of a cation current and associated current noise in entorhinal-cortex layer-II neurons. Journal of Neurophysiology, 88, 1197–1211.
Sigworth, F. J. (1980). The variance of sodium current fluctuations at the node of ranvier. Journal of Physiology (London), 307, 97–129.
Softky, W. R., & Koch, C. (1993). The highly irregular firing of cortical cells is inconsistent with temporal integration of random epsps. Journal of Neuroscience, 13(1), 334–350.
Stopfer, M., Bhagavan, S., Smith, B. H., & Laurent, G. (1997). Impared odor discrimination on desynchronization of odor-encoding neural assemblies. Nature, 390, 70–74.
Strogatz, S. H. (1994). Nonlinear Dynamics and Chaos. Reading MA: Addison Wesley.
Tateno, T., & Pakdaman, K. (2004). Random dynamics of the Morris-Lecar neural model. Chaos, 14, 511–530.
Thomas, P. J., & Lindner, B. (2019). Phase descriptions of a multidimensional Ornstein-Uhlenbeck process. Physical Review E, 99, 062221.
Uhlenbeck, G. E., & Ornstein, L. S. (1930). On the theory of brownian motion. Physical Review, 36, 823–841.
Van Kampen, N. G. (2011). Stochastic Processes in Physics and Chemistry. North-Holland Personal Library.
White, J., Rubinstein, J., & Kay, A. (2000). Channel noise in neurons. Trends in Neurosciences, 23, 131–137.
White, J. A., & Haas, J. S. (2001). Intrinsic noise from voltage-gated ion channels: Effects on dynamics and reliability in intrinsically oscillatory neurons. In Handbook of Biological Physics, 4, 257–278.
White, J. A., Klink, R., Alonso, A., & Kay, A. R. (1998). Noise from voltage-gated ion channels may influence neuronal dynamics in the entorhinal cortex. Journal of Neurophysiology, 80, 262–269.
White, J. A., Rubinstein, J. T., & Kay, A. R. (2000). Channel noise in neurons. Trends in Neurosciences, 23(3), 131–137.
Wiesenfeld, K., & Moss, F. (1995). Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature, 373, 33–36.
Yarom, Y., & Hounsgaard, J. (2011). Voltage fluctuations in neurons: Signal or noise? Physiological Reviews, 91, 917–929.
Zhang, F., Wang, L.-P., Brauner, M., Lewwald, J. F., Kay, K., Watzke, N., et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature, 446, 633–641.
Zhang, K. (1996). Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. Journal of Neuroscience, 16, 2112–2126.
Acknowledgements
This work was partially supported by the National Science Foundation grant DMS-1608077 (HGR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Pulin Gong
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
Intrinsic and resonant oscillatory properties of 2D linear systems
Consider
where a, b, c and d are constants, \(\omega = 2 \pi f / 1000 > 0\) is the input frequency and \(A_{in} \ge 0\) is the input amplitude. The prime sign represents the derivative with respect to t. The units of t are ms and the units of f are Hz.
1.1 Intrinsic oscillations
The characteristic polynomial for the corresponding homogeneous system (\(A_{in} = 0\)) is given by
The eigenvalues are given by
and the natural (intrinsic) frequency of the (damped) oscillations (in Hz if t has units of ms) is given by
assuming \((a-d)^2+4 b c < 0\).
1.2 Resonance and the impedance amplitude profile
The impedance amplitude profile \(Z(\omega )\) for system (10)-(11) is the magnitude
of the complex valued coefficient of the particular solution to the system
For 1D system, these quantities are given, respectively, by
and
The resonance frequency \(f_{res}\) (in Hz if t has units of ms) is the frequency at which Z reaches its maximum
1.3 Response to constant inputs
The equilibrium solution to system (10) for a constant input \(A_{in}\) (i.e., \(\omega = 0\)) is given by
The eigenvectors are given by
The solution satisfying the initial conditions \([x(0)\ \ y(0)]^T = [x_0\ \ y_0]^T\) is given by
where
For 1D systems (\(b = 0\)),
and
where \(x(0) = x_0\)
Ornstein-uhlenbeck (OU) process
1.1 One-dimensional OU process
The 1D OU process Uhlenbeck and Ornstein (1930) is described by the following linear stochastic differential equation
where \(a>0\), I and \(\sigma\) are constants and \(\eta (t)\) is zero-mean and \(\delta\)-correlated Gaussian white noise. The parameter a is the inverse of the time constant and measure the strength by which the system reacts to perturbations. The parameter \(\sigma\) measures the intensity of the noise. The quotient \(I/\alpha\) is the asymptotic mean.
Using standard methods Risken (1989); Gardiner (1985) one can compute the solution satisfying \(X(0) = x_0\), which is the sum of a deterministic function with the form (24) and an integral of a deterministic function with respect to a Wiener process. The solution is normally distributed with mean and variance given, respectively by
and
1.2 Higher-dimensional OU process
The multivariate OU process Uhlenbeck and Ornstein (1930) is described by the following linear stochastic differential equation
where X is an n-dimensional vector, A is an \(n \times n\) matrix, B is an n-dimensional vector, \(\Sigma\) is an \(n \times m\) matrix and H is a vector of independent zero-mean and \(\delta\)-correlated Gaussian white noise components. Using standard methods Risken (1989); Gardiner (1985) one can compute the solution satisfying \(X(0) = x_0\). The solution is normally distributed. The mean is given by
and the covariance matrix is given by
Under certain conditions, the covariance matrix corresponding to the stationary solutions reads
Rights and permissions
About this article
Cite this article
Pena, R.F.O., Rotstein, H.G. Oscillations and variability in neuronal systems: interplay of autonomous transient dynamics and fast deterministic fluctuations. J Comput Neurosci 50, 331–355 (2022). https://doi.org/10.1007/s10827-022-00819-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-022-00819-7