Abstract
An important aspect in the development of small molecules as drugs or agrochemicals is their systemic availability after intravenous and oral administration. The prediction of the systemic availability from the chemical structure of a potential candidate is highly desirable, as it allows to focus the drug or agrochemical development on compounds with a favorable kinetic profile. However, such predictions are challenging as the availability is the result of the complex interplay between molecular properties, biology and physiology and training data is rare. In this work we improve the hybrid model developed earlier (Schneckener in J Chem Inf Model 59:4893–4905, 2019). We reduce the median fold change error for the total oral exposure from 2.85 to 2.35 and for intravenous administration from 1.95 to 1.62. This is achieved by training on a larger data set, improving the neural network architecture as well as the parametrization of mechanistic model. Further, we extend our approach to predict additional endpoints and to handle different covariates, like sex and dosage form. In contrast to a pure machine learning model, our model is able to predict new end points on which it has not been trained. We demonstrate this feature by predicting the exposure over the first 24 h, while the model has only been trained on the total exposure.








Similar content being viewed by others
Notes
Even though the name Pharmacokinetics implies that the field is only concerned with pharmaceutical substances, the field is concerned with all types of xenobiotic substances, see https://en.wikipedia.org/wiki/Pharmacokinetics.
References
Schneckener S et al (2019) Prediction of oral bioavailability in rats: transferring insights from in vitro correlations to (deep) machine learning models using in silico model outputs and chemical structure parameters. J Chem Inf Model 59:4893–4905
Tian S, Li Y, Wang J, Zhang J, Hou T (2011) ADME evaluation in drug discovery. 9. Prediction of oral bioavailability in humans based on molecular properties and structural fingerprints. Mol Pharm 8:841–851. https://doi.org/10.1021/mp100444g
Falcón-Cano G, Molina C, Cabrera-Pérez MÁ (2020) Adme prediction with knime: development and validation of a publicly available workflow for the prediction of human oral bioavailability. J Chem Inf Model 60:2660
Wu Z et al (2019) A comprehensive survey on graph neural networks. IEEE Trans Neural Netw Learn Syst 32:4
Zhang Z, Cui P, Zhu W (2022) Deep learning on graphs: a survey. IEEE Trans Knowl Data Eng 34:249–270
Gilmer J, Schoenholz SS, Riley PF, Vinyals O, Dahl GE (2017) Neural message passing for quantum chemistry
Montanari F, Kuhnke L, Ter Laak A, Clevert D-A (2020) Modeling physico-chemical ADMET endpoints with multitask graph convolutional networks. Molecules 25:44
Rodgers T, Leahy D, Rowland M (2005) Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci 94:1259–1276
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257
Göller AH et al (2020) Bayer’s in silico ADMET platform: a journey of machine learning over the past two decades. Drug Discov Today 25:1702–1709
Open systems pharmacology suite. https://github.com/Open-Systems-Pharmacology/Suite/releases/tag/v8.0
Duvenaud D et al (2015) Convolutional networks on graphs for learning molecular fingerprints. http://arxiv.org/abs/1509.09292. Comment: 9 pages, 5 figures. To appear in Neural Information Processing Systems (NIPS)
Ramsundar B et al (2019) Deep learning for the life sciences (O’Reilly Media). https://www.amazon.com/Deep-Learning-Life-Sciences-Microscopy/dp/1492039837
Ackermann S, Schawinski K, Zhang C, Weigel AK, Turp MD (2018) Using transfer learning to detect galaxy mergers. Mon Not R Astron Soc 479:415
Kim HE et al (2022) Transfer learning for medical image classification: a literature review. BMC Med Imaging 22:69
Farrens S, Lacan A, Guinot A, Vitorelli AZ (2022) Deep transfer learning for blended source identification in galaxy survey data. Astron Astrophys 657:A98
Daga PR, Bolger MB, Haworth IS, Clark RD, Martin EJ (2018) Physiologically based pharmacokinetic modeling in lead optimization. 1. Evaluation and adaptation of gastroplus to predict bioavailability of medchem series. Mol Pharm 15:821–830. https://doi.org/10.1021/acs.molpharmaceut.7b00972
Ilievski I, Akhtar T, Feng J, Shoemaker CA (2016) Efficient hyperparameter optimization of deep learning algorithms using deterministic rbf surrogates
Naga D, Parrott N, Ecker GF, Olivares-Morales A (2022) Evaluation of the success of high-throughput physiologically based pharmacokinetic (HT-PBPK) modeling predictions to inform early drug discovery. Mol Pharm 19:2203–2216
Jones HM et al (2015) Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther 97:247–262
Buck SSD et al (2007) Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos 35:1766–1780
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Maharaj AR, Edginton AN (2014) Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol 3:148
Jones HM et al (2011) Simulation of human intravenous and oral pharmacokinetics of 21 diverse compounds using physiologically based pharmacokinetic modelling. Clin Pharmacokinet 50:331–347
Wang Y et al (2019) Model-informed drug development: current US regulatory practice and future considerations. Clin Pharmacol Ther 105:899–911
Mayr A, Klambauer G, Unterthiner T, Hochreiter S (2016) Deeptox: toxicity prediction using deep learning. Front Environ Sci 3:80
Fabian B et al (2020) Molecular representation learning with language models and domain-relevant auxiliary tasks. CoRR arXiv:2011.13230
Gómez-Bombarelli R et al (2018) Automatic chemical design using a data-driven continuous representation of molecules. ACS Central Sci 4:268–276. https://doi.org/10.1021/acscentsci.7b00572
Winter R, Montanari F, Noé F, Clevert D-A (2019) Learning continuous and data-driven molecular descriptors by translating equivalent chemical representations. Chem. Sci. 10:1692–1701. https://doi.org/10.1039/C8SC04175J
Winter R et al (2019) Efficient multi-objective molecular optimization in a continuous latent space. Chem. Sci. 10:8016–8024. https://doi.org/10.1039/C9SC01928F
Obrezanova O et al (2022) Prediction of in vivo pharmacokinetic parameters and time-exposure curves in rats using machine learning from the chemical structure. Mol Pharm 19:1488–1504
Kingma DP, Welling M (2019) An introduction to variational autoencoders. Found Trends Mach Learn 12:307
Papamakarios G, Nalisnick E, Rezende DJ, Mohamed S, Lakshminarayanan B (2019) Normalizing flows for probabilistic modeling and inference. J Mach Learn Res 22(57):1–64
Kobyzev I, Prince SJD, Brubaker MA (2021) Normalizing flows: an introduction and review of current methods. IEEE Trans Pattern Anal Mach Intell 43:3964–3979
Rackauckas C et al (2020) Universal differential equations for scientific machine learning
Raissi M, Perdikaris P, Karniadakis GE (2017) Physics informed deep learning (part I): data-driven solutions of nonlinear partial differential equations. CoRR arXiv:1711.10561
Raissi M, Perdikaris P, Karniadakis GE (2017) Physics informed deep learning (part II): data-driven discovery of nonlinear partial differential equations. CoRR arXiv:1711.10566
Rackauckas C et al (2019) Diffeqflux.jl-a julia library for neural differential equations. arXiv preprint arXiv:1902.02376
Merkelbach K et al (2022) Hybridml: open source platform for hybrid modeling. Comput Chem Eng 160:107736
Kidger P (2021) On neural differential equations. Ph.D. thesis, University of Oxford
Ashukha A, Lyzhov A, Molchanov D, Vetrov D (2020) Pitfalls of in-domain uncertainty estimation and ensembling in deep learning. arXiv e-prints arXiv:2002.06470
Valentin Jospin L, Buntine W, Boussaid F, Laga H, Bennamoun M (2020) Hands-on bayesian neural networks—a tutorial for deep learning users. arXiv e-prints arXiv:2007.06823
Gawlikowski J et al (2021) A survey of uncertainty in deep neural networks. CoRR arXiv:2107.03342
Gruber A et al. Prediction of human pharmacokinetics from chemical structure: combining mechanistic modeling with machine learning. J Pharm Sci (Submitted)
Peters SA (2012) Physiologically-based pharmacokinetic (pbpk) modeling and simulations. http://search.ebscohost.com/login.aspx?direct=true &scope=site &db=nlebk &db=nlabk &AN=448024. Description based upon print version of record
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Physiologically based pharmacokinetic models
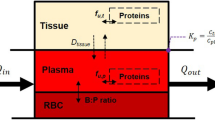
Physiologically based pharmacokinetic (PBPK) models are ordinary differential equation models describing how a substance, e.g. a drug, is absorbed, distributed, metabolized, and excreted in an organism. For the reader not familiar with PBPK models we provide a brief overview over the basic concepts, building blocks and equations forming a PBPK model. For more details we refer to [45].
In PBPK models physiological organs and tissues are represented by compartments. The transport of substance via the blood is modeled by balance equations of the form
where \(C_i\) denotes the compound concentration in the compartment i, \(V_i\) its volume, \(Q_i\) the blood flow, \(P_i\) the partition coefficient between blood and tissue, and \(C_{art}\) the compound concentration in arterial blood, which is governed by
To describe dissolution, absorption, metabolism and excretion, as well as additional distribution mechanism the Eqs. 5 and 6 need to be extended. For example, dissolution and absorption in a single GIT compartment is described by the following equations:
Equation 7 describes concentration in the GIT tissue \(C_g\), which is sourced by a linear absorption process from the GIT lumen. Equation 8 is describes the compound concentration in the GIT lumen \(C_{lum}\), which is sourced by the dissolved compound \(C_{dis}\). Equation 9 is the Noyse-Withney equation describing the dissolution of the compound in the GIT lumen, with K being a compound dependent constant, \(C_0\) is the total amount of compound administered divided by the administered volume and \(C_s\) is the solubility, i.e. the compound concentration the GIT lumen at (thermal) equilibrium. Metabolism is described by the Michaelis–Menten-Kinetics, which for \(C\ll K_m\) can be linearized:
The constants \(V_{max}\) and \(K_M\) depend on the compound and the metabolizing enzyme and control the speed and saturation of metabolism. We assume a single generic metabolizing enzyme, hence in our hybrid model hepatic clearance is fully characterized by the rate \(\frac{V_{max}}{K_M}\).
An active P-gp like transport via membrane proteins, assuming a constant protein concentration, follows also a Michaelis–Menten-Kinetics
As for the metabolism, the constants \(V_{max}\) and \(K_M\) control the speed and saturation of the transport are compound and are transport protein dependent. For our purpose it is sufficient to set \(K_M=1\, \mathrm {\frac{\mu mol}{L}}\), i.e. use the OSP default value, hence the transport is parametrized by its maximal velocity \(V_{max}\).
Appendix 2: Validation of property constraints
In Fig. 9 the distribution of predicted molecule properties of the test set are shown together with the maximal and minimal values in the surrogate training data set. All predicted molecule properties lie within in the surrogates training range, confirming the effectiveness of the penalized loss described in “Training strategy” section. Note that for \(V_{max}\) and FU we used heavy tailed distributions for generating the surrogate training data, resulting in the large range shown in Fig. 9. For the FU this results in unphysiological values \(>1\), for which the equations of the PBPK model are still defined. But in practice the property net does not predict a \(FU >1\). Furthermore, to increase the flexibility of our clearance model we increased the maximal allowed value for the GFR fraction from 1 to 5.25.
Appendix 3: A posteriori surrogate validation
Simulation vs surrogate predictions for the predicted properties of the compounds in our test set for \(\mathrm {AUC_{PO}}\; \)(left), \(\mathrm {AUC_{IV}}\; \)(center) and \(\mathrm {C_{max,PO}}\; \)(right). The accuracy is a bit smaller compared to the estimate on the simulation test set, but still significantly better than the accuracy of the hybrid model, hence the accuracy of the surrogate is sufficient
Hybrid model test set predictions using the full PBPK model instead of the surrogate predictions for the predicted properties of the compounds. The accuracy for the three end-points \(\mathrm {AUC_{PO}}\; \)(left), \(\mathrm {AUC_{IV}}\; \)(center) and \(\mathrm {C_{max,PO}}\; \)(right) is similar to the accuracy when using the surrogate. Demonstrating the accuracy of the surrogate model
We can validate the surrogate model a posteriori by predicting the training targets of our hybrid model using the PBPK model instead of the surrogate. Figure 10 shows the predictions obtained using the PBPK model vs those obtained using the surrogate. The accuracy is not as good as expected from the analysis in “Surrogate” section, but still accurate enough to be used in the hybrid model, the mfce of the surrogate (\(1.2-1.4\)) is clearly better than the mfce of the hybrid model (\(mfce\gtrsim 1.6\)). Additionally, Fig. 11 shows the predictions using the full PBPK vs the observed values. These predictions are almost as accurate as those using the surrogate model. A maximal difference of 0.24 in the mfce can be observed, and no additional features are visible. This highlights again the accuracy of the used surrogate model.
Appendix 4: Charge state dependence of model performance
Dependence of the hybrid models accuracy on the compounds charge state at \(pH=7.4\), i.e the pH value of blood. Shown are the three endpoints \(\mathrm {AUC_{PO}}\; \)(left), \(\mathrm {AUC_{IV}}\; \)(center) and \(\mathrm {C_{max,PO}}\; \)(right) for the case male rat and solution. For neutral compounds the predictions are most accurate, followed by positively and negatively charged compounds. For zwitterions the accuracy is significantly worse, but here the number of compounds is too low for a reliable estimate of the accuracy
We check for a potential dependence of the model accuracy on the charge state in Fig. 12. We evaluate the performance for male rats when a solution is used. As charge states can reliably be predicted, we use predicted charge states at the pH of blood (\(pH=7.4\)). We observe the best performance neutral compounds, and a worse performance for positively and negatively charged compounds. But, in all three cases we achieve \(mfce<3\), so predictions are accurate enough to guide decisions. For zwitterions the mfce for \(\mathrm {AUC_{PO}}\; \)and \(\mathrm {C_{max,PO}}\; \)is larger than 3, but here only very few compounds are in our test set.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Führer, F., Gruber, A., Diedam, H. et al. A deep neural network: mechanistic hybrid model to predict pharmacokinetics in rat. J Comput Aided Mol Des 38, 7 (2024). https://doi.org/10.1007/s10822-023-00547-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10822-023-00547-9