Abstract
Purpose
To analyze the fertilization, developmental, and pregnancy potentials in oocytes with narrow perivitelline space.
Methods
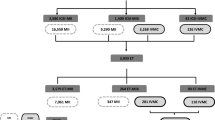
Perivitelline space (PVS) of oocytes was evaluated at the time of ICSI, and those without sufficient PVS were judged as oocytes with narrow PVS (NPVS oocytes), and those with sufficient PVS formation were judged as oocytes with non-narrow PVS (non-NPVS oocytes). The analysis included 634 NPVS oocytes from 278 cycles and 12,121 non-NPVS oocytes from 1698 cycles. The fertilization and developmental potentials of NPVS and non-NPVS oocytes were compared by calculating odds ratios using a mixed-effects logistic regression model. We also compared the embryo transfer outcomes of those used for single vitrified-warmed blastocyst transfer after developing into the blastocyst stage.
Results
NPVS oocytes had higher odds ratios for degeneration (adjusted odds ratio [aOR], 1.555; 95% confidence interval [CI], 1.096–2.206; p = 0.0133) and 0PN (aOR, 1.387; 95% CI, 1.083–1.775; p = 0.0095), resulting in a lower 2PN rate (aOR, 0.761; 95% CI, 0.623–0.929; p = 0.0072). Even embryos with confirmed 2PN had lower odds ratios for cleavage (aOR, 0.501; 95% CI, 0.294–0.853; p = 0.0109) and blastocyst development (Gardner criteria; CC–AA) rates (aOR, 0.612; 95% CI, 0.476–0.788; p = 0.0001). Blastocysts developed from NPVS oocytes had significantly lower odds ratios for clinical pregnancy (aOR, 0.435; 95% CI, 0.222–0.854; p = 0.0156) than those developed from non-NPVS oocytes.
Conclusions
Oocytes with NPVS have low fertilization and developmental potential, as well as low likelihood of pregnancy.


Similar content being viewed by others
References
Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, Sapienza F, Baroni E, Litwicka K, Greco E. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–700. https://doi.org/10.1016/j.fertnstert.2007.09.024.
Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod. 2001;16:1714–8. https://doi.org/10.1093/humrep/16.8.1714.
Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–15. https://doi.org/10.1016/s1472-6483(10)61187-x.
Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, Guglielmino A. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–7. https://doi.org/10.1016/s1472-6483(10)60656-6.
Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod Biomed Online. 2006;12:507–12. https://doi.org/10.1016/s1472-6483(10)62006-8.
Yu EJ, Ahn H, Lee JM, Jee BC, Kim SH. Fertilization and embryo quality of mature oocytes with specific morphological abnormalities. Clin Exp Reprod Med. 2015;42:156–62. https://doi.org/10.5653/cerm.2015.42.4.156.
Setti AS, Figueira RC, Braga DP, Colturato SS, Iaconelli A Jr, Borges E Jr. Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;159:364–70. https://doi.org/10.1016/j.ejogrb.2011.07.031.
Ashrafi M, Karimian L, Eftekhari-Yazdi P, Hasani F, Arabipoor A, Bahmanabadi A, Akhond MR. Effect of oocyte dysmorphisms on intracytoplasmic sperm injection cycle outcomes in normal ovarian responders. J Obstet Gynaecol Res. 2015;41:1912–20. https://doi.org/10.1111/jog.12818.
Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24:263–70. https://doi.org/10.1007/s10815-007-9130-0.
Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15:427–30. https://doi.org/10.1093/humrep/15.2.427.
Ebner, T. Extracytoplasmic markers of human oocyte quality. J Mamm Ova Res. 2009;26:18–25. Retrieved from https://fa.jmor.jp/pdf/26/1/026010018.pdf. Accessed on 10 Aug 2023.
Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, Tews G. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83:1635–40. https://doi.org/10.1016/j.fertnstert.2005.02.009.
Otsuki J, Iwasaki T, Katada Y, Tsutsumi Y, Tsuji Y, Furuhashi K, Kokeguchi S, Shiotani M. A higher incidence of cleavage failure in oocytes containing smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2018;35:899–905. https://doi.org/10.1007/s10815-018-1119-3.
Faramarzi A, Khalili MA, Ashourzadeh S. Oocyte morphology and embryo morphokinetics in an intra-cytoplasmic sperm injection programme. Is there a relationship? Zygote. 2017;25:190–6. https://doi.org/10.1017/S0967199417000041.
Balaban B, Ata B, Isiklar A, Yakin K, Urman B. Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos. Hum Reprod. 2008;23:1778–85. https://doi.org/10.1093/humrep/den127.
Takahashi H, Otsuki J, Yamamoto M, Saito H, Hirata R, Habara T, Hayashi N. Clinical outcomes of MII oocytes with refractile bodies in patients undergoing ICSI and single frozen embryo transfer. Reprod Med Biol. 2019;19:75–81. https://doi.org/10.1002/rmb2.12305.
Setti AS, Figueira RC, de Almeida Ferreira Braga DP, Azevedo MC, Iaconelli A Jr, Borges E Jr. Oocytes with smooth endoplasmic reticulum clusters originate blastocysts with impaired implantation potential. Fertil Steril. 2016;106:1718–24. https://doi.org/10.1016/j.fertnstert.2016.09.006.
Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7. https://doi.org/10.1093/humrep/deh258.
Saito H, Otsuki J, Takahashi H, Hirata R, Habara T, Hayashi N. A higher incidence of smooth endoplasmic reticulum clusters with aromatase inhibitors. Reprod Med Biol. 2019;18:384–9. https://doi.org/10.1002/rmb2.12296.
Sauerbrun-Cutler MT, Vega M, Breborowicz A, Gonzales E, Stein D, Lederman M, Keltz M. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res. 2015;8:5. https://doi.org/10.1186/s13048-014-0111-5.
Taheri F, Alemzadeh Mehrizi A, Khalili MA, Halvaei I. The influence of ovarian hyperstimulation drugs on morphometry and morphology of human oocytes in ICSI program. Taiwan J Obstet Gynecol. 2018;57:205–10. https://doi.org/10.1016/j.tjog.2018.02.007.
Talbot P, Dandekar P. Perivitelline space: does it play a role in blocking polyspermy in mammals? Microsc Res Tech. 2003;61:349–57. https://doi.org/10.1002/jemt.10348.
Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J. Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod. 1998;13:3425–30. https://doi.org/10.1093/humrep/13.12.3425.
Ferrarini Zanetti B, Paes de Almeida Ferreira Braga D, Souza Setti A, de CássiaSávioFigueira R, Iaconelli A Jr, Borges E Jr. Is perivitelline space morphology of the oocyte associated with pregnancy outcome in intracytoplasmic sperm injection cycles? Eur J Obstet Gynecol Reprod Biol. 2018;231:225–9. https://doi.org/10.1016/j.ejogrb.2018.10.053.
Hassa H, Aydın Y, Taplamacıoğlu F. The role of perivitelline space abnormalities of oocytes in the developmental potential of embryos. J Turk Ger Gynecol Assoc. 2014;15:161–3. https://doi.org/10.5152/jtgga.2014.13091.
Farhi J, Nahum H, Weissman A, Zahalka N, Glezerman M, Levran D. Coarse granulation in the perivitelline space and IVF-ICSI outcome. J Assist Reprod Genet. 2002;19:545–9. https://doi.org/10.1023/a:1021243530358.
Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–5. https://doi.org/10.1093/humrep/12.8.1750.
Braga DP, Setti AS, Figueira Rde C, Machado RB, Iaconelli A Jr, Borges E Jr. Influence of oocyte dysmorphisms on blastocyst formation and quality. Fertil Steril. 2013;100:748–54. https://doi.org/10.1016/j.fertnstert.2013.05.021.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3. https://doi.org/10.1093/humrep/13.12.3431.
Tartia AP, Rudraraju N, Richards T, Hammer MA, Talbot P, Baltz JM. Cell volume regulation is initiated in mouse oocytes after ovulation. Development. 2009;136:2247–54. https://doi.org/10.1242/dev.036756.
Clarke HJ. Transzonal projections: essential structures mediating intercellular communication in the mammalian ovarian follicle. Mol Reprod Dev. 2022;89:509–25. https://doi.org/10.1002/mrd.23645.
Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56:909–18. https://doi.org/10.1387/ijdb.120135gv.
Inoue A, Akiyama T, Nagata M, Aoki F. The perivitelline space-forming capacity of mouse oocytes is associated with meiotic competence. J Reprod Dev. 2007;53:1043–52. https://doi.org/10.1262/jrd.19064.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. https://doi.org/10.1016/s0015-0282(00)00518-5.
Rosen MP, Shen S, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Oocyte degeneration after intracytoplasmic sperm injection: a multivariate analysis to assess its importance as a laboratory or clinical marker. Fertil Steril. 2006;85:1736–43. https://doi.org/10.1016/j.fertnstert.2005.12.017.
Ebner T, Moser M, Sommergruber M, Puchner M, Wiesinger R, Tews G. Developmental competence of oocytes showing increased cytoplasmic viscosity. Hum Reprod. 2003;18:1294–8. https://doi.org/10.1093/humrep/deg232.
Krause I, Pohler U, Grosse S, Shebl O, Petek E, Chandra A, Ebner T. Characterization of the injection funnel during intracytoplasmic sperm injection reflects cytoplasmic maturity of the oocyte. Fertil Steril. 2016;106:1101–6. https://doi.org/10.1016/j.fertnstert.2016.06.015.
Iwayama H, Hochi S, Yamashita M. Low stretching ability of human oolemma during piezo-ICSI as a risk factor on post-injection survival and implantation. J Mamm Ova Res. 2010;27:150–6. https://doi.org/10.1274/jmor.27.150.
Jiang Y, Song G, Yuan J, Zhang X. ICSI with all oocytes recurrent metaphase I characterized by absence perivitelline space. Open J Obstet Gynecol. 2021;11:1112–6. https://doi.org/10.4236/ojog.2021.119104.
Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19:1587–90. https://doi.org/10.1093/humrep/deh236.
Sugimoto H, Kida Y, Miyamoto Y, Kitada K, Matsumoto K, Saeki K, Taniguchi T, Hosoi Y. Growth and development of rabbit oocytes in vitro: effect of fetal bovine serum concentration on culture medium. Theriogenology. 2012;78:1040–7. https://doi.org/10.1016/j.theriogenology.2012.04.007.
Inoue T, Yamashita Y, Tsujimoto Y, Yamamoto S, Taguchi S, Hirao K, Uemura M, Ikawa K, Miyazaki K. The association of follicular fluid volume with human oolemma stretchability during intracytoplasmic sperm injection. Clin Exp Reprod Med. 2017;44:126–31. https://doi.org/10.5653/cerm.2017.44.3.126.
Inoue T, Taguchi S, Hirao K, Tsujimoto Y, Yamamoto S, Uemura M, Miyazaki K, Yamashita Y. Association of follicular fluid volume with membrane stretchability of human metaphase II oocytes following the gonadotropin-releasing hormone agonist protocol during intracytoplasmic sperm injection. Reprod Med Biol. 2018;17:283–8. https://doi.org/10.1002/rmb2.12102.
Zander-Fox D, Lam K, Pacella-Ince L, Tully C, Hamilton H, Hiraoka K, McPherson NO, Tremellen K. PIEZO-ICSI increases fertilization rates compared with standard ICSI: a prospective cohort study. Reprod Biomed Online. 2021;43:404–12. https://doi.org/10.1016/j.rbmo.2021.05.020.
Caddy M, Popkiss S, Weston G, Vollenhoven B, Rombauts L, Green M, Zander-Fox D. PIEZO-ICSI increases fertilization rates compared with conventional ICSI in patients with poor prognosis. J Assist Reprod Genet. 2023;40:389–98. https://doi.org/10.1007/s10815-022-02701-y.
Hiraoka K, Kitamura S. Clinical efficiency of Piezo-ICSI using micropipettes with a wall thickness of 0.625 μm. J Assist Reprod Genet. 2015;32:1827–33. https://doi.org/10.1007/s10815-015-0597-9.
Hiraoka K, Isuge M, Kamada Y, Kaji T, Suhara T, Kuga A, Ohuchi K, Hayashi M, Kawai K. Piezo-ICSI for human oocytes. J Vis Exp. 2021;(170). https://doi.org/10.3791/60224.
Morimoto T, Maekawa T, Mizuta S, Matsubayashi H, Takeuchi T, Hata Y, Ishikawa T. Optimal puncture position for ICSI can be detected by image analysis using Local Binary Pattern. Reprod Biomed Online. 2023;46:46–53. https://doi.org/10.1016/j.rbmo.2022.09.011.
Derrick R, Hickman C, Oliana O, Wilkinson T, Gwinnett D, Whyte LB, Carby A, Lavery S. Perivitelline threads associated with fragments in human cleavage stage embryos observed through time-lapse microscopy. Reprod Biomed Online. 2017;35:640–5. https://doi.org/10.1016/j.rbmo.2017.08.026.
Kellam L, Pastorelli LM, Bastida AM, Senkbeil A, Montgomery S, Fishel S, Campbell A. Perivitelline threads in cleavage-stage human embryos: observations using time-lapse imaging. Reprod Biomed Online. 2017;35:646–56. https://doi.org/10.1016/j.rbmo.2017.09.004.
Van Blerkom J, Alikani M. Perivitelline threads: an overlooked feature of cleavage-stage human embryos or an epiphenomenon in search of a function? Reprod Biomed Online. 2017;35:625–6. https://doi.org/10.1016/j.rbmo.2017.10.105.
Wu DH, Reynolds K, Maxwell R, Lindheim SR, Aubuchon M, Thomas MA. Age does not influence the effect of embryo fragmentation on successful blastocyst development. Fertil Steril. 2011;95:2778–80. https://doi.org/10.1016/j.fertnstert.2011.05.024.
Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15:2634–43. https://doi.org/10.1093/humrep/15.12.2634.
Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9. https://doi.org/10.1093/humrep/15.12.2634.
Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42. https://doi.org/10.1016/s0015-0282(99)00092-8.
Rudnicka E, Kunicki M, Calik-Ksepka A, Suchta K, Duszewska A, Smolarczyk K, Smolarczyk R. Anti-Müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci. 2021;22:12507. https://doi.org/10.3390/ijms222212507.
Xiao S, Li Y, Li T, Chen M, Xu Y, Wen Y, Zhou C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J Clin Endocrinol Metab. 2014;99:E1015–21. https://doi.org/10.1210/jc.2013-4177.
Acknowledgements
We thank the staff of Takahashi Women’s Clinic. We also would like to thank the Chiba University Academic English consultation service for the English language review. Furthermore, we would like to show our greatest appreciation to Dr. Yuki Shiko at the Clinical Research Center, Chiba University Hospital for advice on statistical analysis.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Masashi Shioya, Miki Okabe-Kinoshita, Tatsuya Kobayashi, Maki Fujita, and Keiichi Takahashi. The first draft of the manuscript was written by Masahi Shioya, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional review board of Takahashi Women’s Clinic (protocol number: TWC20-001).
Consent to participate
Consent for this study was obtained in the form of opt-out through our clinic website and a bulletin board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shioya, M., Okabe-Kinoshita, M., Kobayashi, T. et al. Human metaphase II oocytes with narrow perivitelline space have poor fertilization, developmental, and pregnancy potentials. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03084-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03084-y