Abstract
Purpose
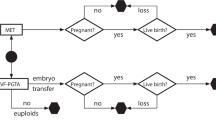
To identify specific likelihoods that an embryo will be classified as appropriate for transfer after preimplantation genetic testing for detection of a monogenic disorder (PGT-M), with or without preimplantation genetic testing for aneuploidy (PGT-A), separated by inheritance pattern.
Methods
Retrospective chart review of 181 selected PGT-M cycles performed at CooperGenomics in 2018 or 2019. For each cycle, the following main outcome data was collected: the number of embryos classified as affected with monogenic disease, the number detected to be chromosomally abnormal, the number that were recombinant, the number that had no result, and if applicable, the number which were aneuploid.
Results
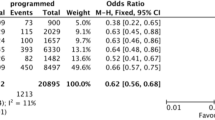
There were significantly fewer embryos appropriate to consider for transfer when PGT-A was included for autosomal recessive and X-linked disorders. There were also fewer for autosomal dominant disorders, though this was not statistically significant. When PGT-A was not included, 45.8% of autosomal dominant, 69% of autosomal recessive, and 47.8% of X-linked embryos were appropriate to consider for transfer. When PGT-A analysis was included, 29% of autosomal dominant, 41% of autosomal recessive, and 22% of X-linked embryos were appropriate to consider for transfer. 96.8% of women elect to include PGT-A when pursuing PGT-M.
Conclusion
This study resulted in specific likelihoods that an embryo would be found appropriate for clinicians and patients to consider for transfer based on the inheritance pattern of the monogenic disease being tested for and whether aneuploidy analysis was included.


Similar content being viewed by others
References
Simpson JL, Kuliev A, Rechitsky S. Overview of preimplantation genetic diagnosis (PGD): historical perspective and future direction [Internet]. Humana Press, New York, NY; 2019 [cited 2019 Feb 26]. p. 23–43. Available from: https://doi.org/10.1007/978-1-4939-8889-1_2
Won SY, Kim H, Lee WS, Kim JW, Shim SH. Pre-implantation genetic diagnosis and pre-implantation genetic screening: two years experience at a single center. Obstet Gynecol Sci [Internet] 2018 [cited 2019 Feb 3];61(1):95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29372155
Brezina PR, Anchan R, Kearns WG. Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J Assist Reprod Genet [Internet] 2016 [cited 2019 Mar 11];33(7):823–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27299602
Zimmerman RS, Eccles J, Jalas C, Treff NR, Scott RT. Molecular testing for preimplantation genetic diagnosis of single gene disorders [Internet]. Humana Press, New York, NY; 2019 [cited 2019 Feb 26]. p. 61–71. Available from: https://doi.org/10.1007/978-1-4939-8889-1_4
Treff NR, Zimmerman RS. Advances in preimplantation genetic testing for monogenic disease and aneuploidy. Annu Rev Genomics Hum Genet [Internet]. 2017;18(1):189–200. https://doi.org/10.1146/annurev-genom-091416-035508.
Geraedts J. Healthy children without fear: reproductive options for patients or couples carrying inherited diseases. EMBO Rep [Internet] 2017 [cited 2019 Mar 11];18(5):666–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28396574
Masbou AK, Friedenthal JB, McCulloh DH, McCaffrey C, Fino ME, Grifo JA, et al. A comparison of pregnancy outcomes in patients undergoing donor egg single embryo transfers with and without preimplantation genetic testing. Reprod Sci [Internet] 2018 [cited 2019 Mar 11];193371911882047. Available from: https://doi.org/10.1177/1933719118820474
Kim TG, Neblett MF, Shandley LM, Omurtag K, Hipp HS, Kawwass JF. National mosaic embryo transfer practices: a survey. Am J Obstet Gynecol [Internet] 2018 [cited 2019 Mar 11];219(6):602.e1–602.e7. Available from: https://www.sciencedirect.com/science/article/pii/S0002937818308226?via%3Dihub
Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid–aneuploid blastocysts. Hum Genet [Internet]. 2017;136(7):805–19. https://doi.org/10.1007/s00439-017-1797-4.
Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril [Internet] 2018 [cited 2019 Feb 3];109(1):77–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29191449
Vaz-de-Macedo C, Harper J. A closer look at expanded carrier screening from a PGD perspective. Hum Reprod [Internet] 2017 [cited 2019 Jun 17];32(10):1951–6. Available from: http://academic.oup.com/humrep/article/32/10/1951/4097721/A-closer-look-at-expanded-carrier-screening-from-a
Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602. https://doi.org/10.1016/j.fertnstert.2016.04.027.
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, Wells D, Gordon T, Stock-Myer S, Willman S, STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071-1079.e7. https://doi.org/10.1016/j.fertnstert.2019.07.1346.
Kushnir VA, Darmon SK, Albertini DF, Barad DH, Gleicher N. Effectiveness of in vitro fertilization with preimplantation genetic screening: a reanalysis of United States assisted reproductive technology data 2011–2012. Fertil Steril [Internet] 2016 [cited 2019 Mar 13];106(1):75–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26952783
Murphy LA, Seidler EA, Vaughan DA, Resetkova N, Penzias AS, Toth TL, et al. To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A). Hum Reprod [Internet] 2019 [cited 2019 Mar 11];34(2):268–75. Available from: https://academic.oup.com/humrep/article/34/2/268/5219186
Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Electronic address: ASRM@asrm.org; Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018 Mar;109(3):429–436. https://doi.org/10.1016/j.fertnstert.2018.01.002
Goldman KN, Nazem T, Berkeley A, Palter S, Grifo JA. Preimplantation genetic diagnosis (PGD) for monogenic disorders: the value of concurrent aneuploidy screening. J Genet Couns [Internet]. 2016;25(6):1327–37. https://doi.org/10.1007/s10897-016-9975-4.
Brezina PR, Kutteh WH, Bailey AP, Ke RW. Preimplantation genetic screening (PGS) is an excellent tool, but not perfect: a guide to counseling patients considering PGS. Fertil Steril [Internet] 2016 [cited 2019 Mar 11];105(1):49–50. Available from: https://www.sciencedirect.com/science/article/pii/S0015028215020063?via%3Dihub
Dahdouh EM, Balayla J, García-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril [Internet] 2015 [cited 2019 Mar 13];104(6):1503–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26385405
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril [Internet] 2014 [cited 2019 Mar 13];101(3):656–663.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24355045
Toft CLF, Ingerslev HJ, Kesmodel US, et al. A systematic review on concurrent aneuploidy screening and preimplantation genetic testing for hereditary disorders: what is the prevalence of aneuploidy and is there a clinical effect from aneuploidy screening? Acta Obstet Gynecol Scand. 2020;99:696–706. https://doi.org/10.1111/aogs.13823.
Brezina PR, Kutteh WH. Clinical applications of preimplantation genetic testing. BMJ [Internet] 2015 [cited 2019 Mar 18];350:g7611. Available from: https://www.bmj.com/content/350/bmj.g7611
Viotti M, Victor AR, Barnes FL, Zouves CG, Besser AG, Grifo JA, Cheng EH, Lee MS, Horcajadas JA, Corti L, Fiorentino F, Spinella F, Minasi MG, Greco E, Munné S. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021;115(5):1212–24. https://doi.org/10.1016/j.fertnstert.2020.11.041.
Insogna IG, Lanes A, Dobson L, Ginsburg ES, Racowsky C, Yanushpolsky E. Blastocyst conversion rate and ploidy in patients with structural rearrangements. J Assist Reprod Genet. 2021. https://doi.org/10.1007/s10815-021-02131-2.
Kalfoglou AL, Scott J, Hudson K. PGD patients’ and providers’ attitudes to the use and regulation of preimplantation genetic diagnosis. Reprod Biomed Online [Internet] 2005 [cited 2019 Mar 18];11(4):486–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16274615
McGowan ML, Burant CJ, Moran R, Farrell R. Patient education and informed consent for preimplantation genetic diagnosis: health literacy for genetics and assisted reproductive technology. Genet Med [Internet] 2009 [cited 2019 Mar 18];11(9):640–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19652605
Munné S, Alikani M, Ribustello L, Colls P, Martínez-Ortiz PA, McCulloh DH, Referring Physician Group. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32(4):743–9. https://doi.org/10.1093/humrep/dex031.
Acknowledgements
This work was conducted by researcher ES to fulfill Master’s degree requirements for Case Western Reserve University’s Genetic Counseling Training Program in Cleveland, Ohio. The authors would like to thank Kevin Cavanagh, PhD, for his contributions to the statistical analyses performed in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stocker, E., Johal, S., Rippel, L. et al. Frequency of embryos appropriate for transfer following preimplantation genetic testing for monogenic disease. J Assist Reprod Genet 39, 2043–2050 (2022). https://doi.org/10.1007/s10815-022-02571-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02571-4